Note: This guideline is currently under review.
Introduction
Aim
Indications
Definition of Terms
Insertion
Assessment of Equipment
Management and Physical Assessment
Special Considerations
Companion Documents
Links
Evidence Table
References
Introduction
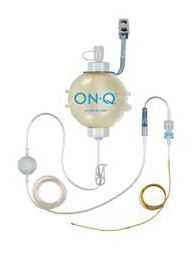
The ON-Q PainBuster® is an elastomeric pump that contains a reservoir of local anaesthetic (LA) that is infused at a constant and preset rate through a catheter that is fenestrated over 4 lengths depending on the catheter used. The catheter can be placed perineurally (alongside a peripheral nerve) or under a surgical incision (wound catheter).
The use of a continuous LA infusion has been shown to reduce post-operative pain and opioid requirement following surgery and may be used as part of a multimodal analgesia strategy. At the time of surgery a catheter is placed alongside a peripheral nerve or beneath the surgical incision to enable the delivery of LA directly where it is needed. The LA is delivered through an On-Q Soaker Catheter to the On-Q Pain Buster system
Alternatively, LA can be delivered through a wound catheter using a syringe driver colour coded yellow to identify it as regional analgesia. The Alaris syringe drivers are required to be programmed (locked) and the syringe refilled by nursing staff on the ward as indicated in the MAR. The syringe pump is heavy and needs to be fixed to a pole which may inhibit patient mobility and physiotherapy.
The ON-Q PainBuster® system is light and can be carried in a small cloth bag for improved patient mobility
Aim
- To provide nursing staff with a standardised guideline to enable safe and appropriate care of children with a Pain Buster for post-operative pain relief.
- To provide guidance on the Pain Busters intended use in post-operative pain relief by delivering a continuous infusion of local anaesthetic directly into the surgical site
- To outline the process of Pain Buster insertion and management throughout the patient journey intraoperatively and will be set up and working prior to transfer from the operating theatre.
Indications
- To provide continuous delivery of LA for the management of moderate to severe pain.
- Pain Buster infusions may be used in conjunction with opioids, when using opioid infusions alone would result in inadequate analgesia.
- Indicated to reduce opioid requirements, minimise opioid tolerance and related nausea, vomiting and constipation when prolonged opioid administration is anticipated.
- Pain Buster infusions may be used alone in some situations along with simple analgesia
Definition of Terms
- Local Anaesthesia: an agent which blocks the conduction of impulses in nerve tissues through sodium channel deactivation
- ON-Q soaker catheter: a tube similar in size to an epidural catheter with fenestrations along a variable length (2.5cm, 6.5cm, 12.5cm and 25cm) and black markings indicating the distance from the most proximal fenestration.
- ON-Q PainBuster®: a small disposable pump that continuously delivers local anaesthetic which is connected to an ON-Q soaker catheter.
The device is for continuous infusion of LA through a wound catheter in patients who are likely to have pain at the site of surgery eg: bone/cartilage donor sites, lower limb surgery and laparotomy. It is also used for continuous infusion of LA perineurally after upper limb or lower limb surgery where blocking a peripheral nerve’s conduction, is likely to reduce postoperative pain.
Insertion
- The soaker catheter is inserted by the surgeon and the ON-Q PainBuster® device filled and attached in the operating theatre by the theatre nursing staff.
- The soaker catheter must not be cut / shortened.
- The ON-Q PainBuster® must be prescribed by the Anaesthetist in the MAR as outlined by the Pain Management Guidelines for Regional Anaesthetic Infusion Blocks

Assessment of Equipment
- The tubing attached to the soaker catheter has a clamp. In order to work, the clamp must be unclamped.
- The pump should be positioned below the catheter site to ensure accurate flow
- The filter vent should not be taped or covered
- The clamp is usually unclamped in PACU unless otherwise ordered.
- The medication labels are to be printed and attached to the pump and lines and checked by two nurses with the MAR and patient details
- The nurse will document when the infusion starts
- At the time of filling, the ON-Q PainBuster® pump is round and firm. It should always be filled to its specified volume as delivery becomes inaccurate if it is under- or over-filled.
- There are three layers: the inner layer is where the anaesthetic agent is placed and does not contain natural rubber latex. The middle layer is made from natural rubber latex. The outer layer is PVC. There is no risk to patients with latex allergies.
- ON-Q PainBuster® used at RCH is pressurized to deliver LA at a specified rate that cannot be adjusted.
- The rate may be 2ml/h or 5 ml/h depending on the pump specification.
- The time that the pump empties will depend on the volume of the ON-Q PainBuster® attached to the soaker catheter. For example, a 100ml ON-Q PainBuster® delivering at 2 ml/h will be empty 50 hours after the clamp is unclamped. A 270ml ON-Q PainBuster® delivering at 5 ml/h will be empty 54 hours after the clamp is unclamped.
- During the infusion the pump will lose shape, the outer layer will become wrinkled and the inner layer will take the appearance of a core as it deflates. It is not possible to determine if the pump is fully discharged (empty) by observing it.
Management and Physical Assessment
Acute management and assessment on return to the ward
- patients require routine post operative clinical observations
- hourly pain assessment
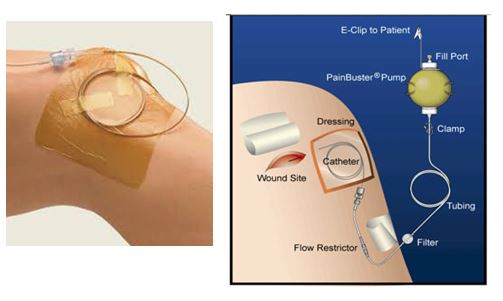
- check pain buster catheter exit site at the skin and the operative wound dressing for excessive leak 4 hourly
- ensure the fenestrations are within the wound (the distance from the most proximal soaker catheter fenestration can be determined by looking at the black markings on the soaker catheter). The first double black lines close together is at 10cm from the most proximal fenestration.
- ensure flow restrictor is taped to patient skin with adhesive dressing to ensure flow rate accuracy (see image below)
- do not tape over the filter
- check catheter is connected to the pump
- check catheter is unclamped
- ensure the pump is not under the bedcovers (to avoid heating as the pump is calibrated to room temperature)
Ongoing management
- Infusion to continue until the date and time specified by the anaesthetist prescriber after which the soaker catheter can be removed and the whole system including the ON-Q PainBuster® be disposed of appropriately
- Assess for LA toxicity (link to Anaesthesia and pain Management regional/epidural)
- Pain assessment – the ON-Q PainBuster® is used as part of a multimodal analgesia regimen and other additional analgesia will often still be necessary.
- Managing complications/troubleshooting, leaking at site, catheter dislodged, pump not infusing, pain increasing despite analgesia medications and pain buster contact CPMS 5773
- If there is leaking at the catheter insertion site it is recommended to apply a pressure dressing and contact CPMS.
Removing the PainBuster® catheter
- Explain the removal technique to patient if age/cognition appropriate and to the carer
- Use Comfort Kids guidelines for procedures
www.rch.org.au/comfortkids/
- Use standard aseptic technique and universal precautions as per guideline
https://www.rch.org.au/policy/policies/Aseptic_Technique/
- Lift the dressing securing the soaker catheter in place and loosen adhesive at the catheter site if present
- Gently pull the soaker catheter out in one smooth movement, holding the catheter close to the exit site at the skin.
- There may be some initial resistance if dermabond has been used to prevent catheter migration. The catheter should be easily removed and is not painful
- If any resistance stop and contact surgical team
- Check the distal end marker to ensure the tip is intact, if any concerns contact the surgical team and CPMS
- Cover site with a clear dressing
- Discharge planning and community-based management.
Some families are happy to remove the catheter with instructions from CPMS. An information sheet outlining removal will be discussed with the family member who will remove the catheter. The CPMS contact phone number is included on the information sheet.
If a patient is discharged with the catheter in situ and the family do not want to remove the catheter, A HITH referral can be made for families in the HITH catchment area
Special Considerations
- infection control
- patient safety alerts
- potential adverse events
Companion Documents
Links
Evidence Table
The
evidence table for this nursing guideline can be found here.
References
Photos supplied with permission from the product specialist and trainer for ON-Q
Please remember to
read the disclaimer.
The development of this nursing guideline was coordinated by Sueann Penrose, Clinical Nurse Consultant, Children's Pain management Service, and approved by the Nursing Clinical Effectiveness Committee. Updated June 2020.