- Platelets are small, disc shaped cells that have a critical role in helping
our blood clot and stop bleeding. When there is a break in the vascular
endothelium, a process of platelet activation occurs and the platelets change
shape and aggregate to form a platelet plug.
- Platelets are commonly transfused to patients with low platelet counts or patients
with platelet dysfunction who are bleeding or at high risk of bleeding.
- All platelet components are leucodepleted and irradiated prior to release from Lifeblood.
Platelet transfusion indications
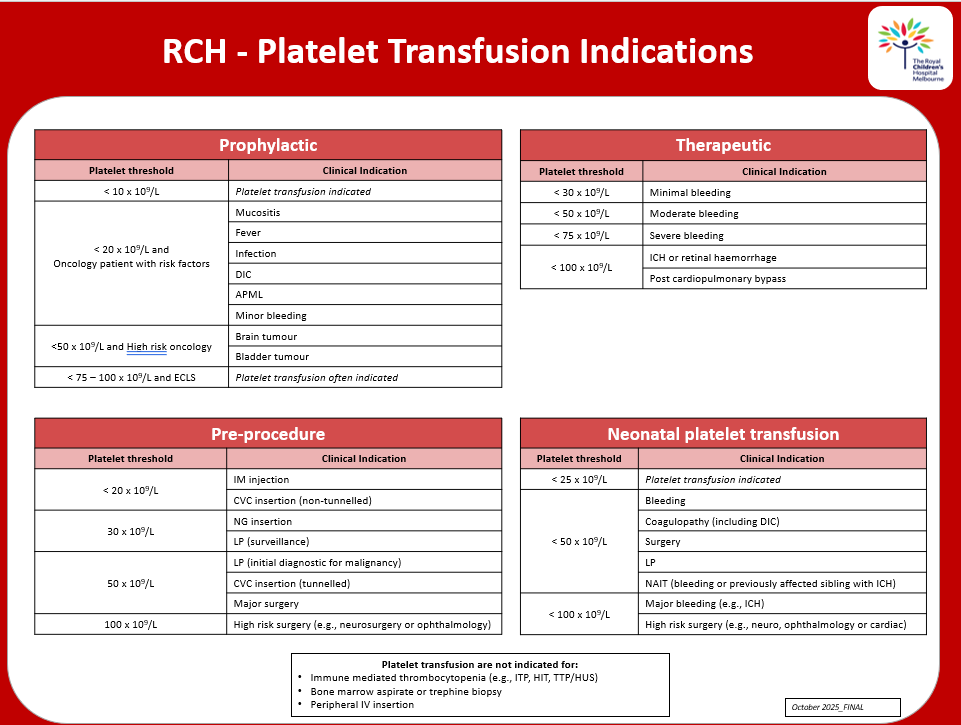
RCH - Platelet transfusion indications poster for download.
![]()
Dose, characteristics of platelet products, administration, compatibility and storage

Platelet dose, administration, compatibility and storage poster for download.
Dose
Platelet product Dose if < 15 kg Dose if > 15 kg
Pooled or apheresis platelets (including pedipaks) 10 mL/kg One adult unit
Types of products available:
Apheresis platelets
Dose of platelets obtained from a single donor and suspended in a mixture of Platelet Additive Solution (PAS) and approximately 40% donor plasma. Apheresis platelets can be used to decrease donor exposure in chronically transfused patients. In patients who rely on platelet support e.g. severe aplastic anaemia, they may be selected when available to reduce the risk of alloimmunisation.
Characteristics:
- Volume (mL) 216 ± 9 (100 mL – 400 mL)
- Platelet count (109/unit) 287 ± 35
Paediatric apheresis platelets (pedipaks)
One unit of apheresis platelets may be divided into three equal packs to create paediatric sized components (pedipaks). This will enable smaller patients requiring small but regular top ups to have exposure to less donor products and minimise product wastage.
Characteristics:
- Volume (mL) 55 ± 2 (40 mL – 60mL)
- Platelet count (109/unit) 71 ±9 (>50)
Pooled platelets in PAS
An adult dose of pooled platelets are obtained from a pool of buffy coats from four donors. These are pooled and re-suspended in PAS to create one unit of pooled platelets.
Certain patient groups may require pooled platelets as the first choice. The ratio of plasma to platelets is less in pooled components than apheresis products and therefore the exposure to plasma is less. This becomes significant for those patient groups who have mild – moderate allergic reaction to apheresis platelets.
Characteristics:
- Volume (mL) 270± 12 (>160)
- Platelet count (109/unit) 264 ± 37
Platelet administration
- The patient should be ready for transfusion prior to picking up platelets from the blood bank. e.g. appropriate IV access and medical order for transfusion.
- Patient identification is required for all blood products to be issued from blood bank
- Complete positive patient and blood product identification prior to transfusion of platelets as per RCH Blood Transfusion - Fresh Blood Products Procedure.
- Care and monitoring of transfused patients, refer to Blood Administration
- Administer via a volumetric pump or syringe driver to ensure accurate volume delivered.
- Standard 170–200-micron filter either in-line or on transfer to syringe.
- Use a new blood administration filter (170 - 200 micron) when administering platelets.
- Do not transfuse though the same blood administration filter after red blood cell transfusion as some platelets may get caught in fibrin strands/debris caught in filter.
Compatibility
- The provision of ABO and Rh(D) identical platelet transfusion is ideal, but not always possible. If ABO compatible components are unavailable, patient age, weight, diagnosis and component availability (pooled vs apheresis) will influence the blood bank decision about what product to supply.
- An ABO incompatible platelet transfusion (group O platelets given to a group A patient) may be associated with clinically significant transfusion reactions, including a positive DAT, red cell haemolysis and even lower platelet survival in some patients.
- Platelet components contain a small number of red cells that could be Rh incompatible with the recipient. Therefore, RhD negative females with childbearing potential should receive platelet transfusions from RhD negative donors. If transfusion of RhD positive product to RhD negative recipient is unavoidable, consider giving Rhesus immunoglobulin (Discuss with haematologist-on-call)
Special platelet transfusions
Platelet Transfusion in patients undergoing stem
cell transplantation
- Where possible, a platelet product compatible
with both donor and recipient should be used. At RCH the platelet product
choice for each transplant recipient will be specified by their transplant
physician and will be listed on the Transplant Protocol
Platelet transfusion in children
with congenital platelet disorders
- Platelet transfusion in rare congenital platelet
disorders such as Bernard-Soulier syndrome, Glanzmann's thrombasthenia, thrombocytopenia
with absent radii (TAR), Wiskott-Aldrich syndrome, Fanconi anaemia,
amegakaryocytic thrombocytopenia can provoke the development of multi-specific
HLA or platelet specific antibodies and they should be used sparingly. They
should be reserved for clinical bleeding or prior to invasive procedures with a
high risk of bleeding.
- Donor exposure should be limited through the use of
apheresis platelets and the risk of alloimmunisation reduced through the use of
leukocyte reduced products.
Platelet transfusion in immune
thrombocytopenia
- Transfused platelets are rapidly destroyed and
should be reserved for cases of life-threatening bleeding.
Special Products
Phenotype
specific for patients with fetomaternal alloimmunisation (FMAIT)
- The infant or fetus with confirmed or suspected
alloimmune thrombocytopenia should be transfused platelets which are negative
for the implicated alloantigen. Platelets negative for the HPA-1a antigen
(implicated in 85% of cases of FMAIT in Caucasian populations) are often
available from ARCBS but may be sourced from interstate. Contact the
haematologist on-call for advice regarding platelet support in this clinical
situation.
HLA matched for immunised refractory patients:
- When patients fail to achieve a significant and
sustained rise in the platelet count following platelet transfusion (platelet
increment) they are said to be 'refractory'. There are clinical and
immunological causes of platelet refractoriness. Clinical causes include fever,
sepsis, bleeding, DIC and some drugs. In these situations, patients may respond
to more frequent platelet transfusions or higher doses of platelets. Patients
undergoing stem cell transplantation, who are multiply transfused, or who have
had prior pregnancy may become refractory to platelet transfusion due to the
development of multispecific HLA or platelet-specific antibodies. These patients
may require platelet support from HLA (Human Leucocyte Antigen) or HPA (Human
Platelet Antigen) matched donors.
- If HLA or HPA matched apheresis platelets are
required, please contact the haematologist on-call for advice and RCH Blood Bank, ph 55829.
IgA deficient:
- IgA
deficient components may be used in patients who have developed an anti-IgA
antibody and who also have recurrent, severe allergic reactions. The FFP is
obtained from donors who have known low titre IgA/IgA deficiency.
Adverse effects
of platelet transfusion
- See section on adverse
effects of transfusion.
- Platelet products are collected from volunteer donors screened
with standard screening tests and have the same risks of infectious
disease transmission as red cell products.