Introduction
Newborn bloodspot screening (NBS) (formally known as newborn screening testing) commenced in Victoria in 1966, when screening was introduced for Phenylketonuria. Since then, screening has expanded to testing for Congenital Hypothyroidism, Cystic Fibrosis, Phenylketonuria, Congenital Adrenal Hyperplasia (CAH) and over 22 other rare conditions. These rare but serious conditions cannot be seen by just looking at the baby. These tests ensure early detection and treatment of these conditions to save lives.
Aim
This guideline provides an outline for medical and nursing staff to perform screening tests for newborn infants at the Royal Children's Hospital.
Definition of Terms
-
Palliated Infant
Palliative care for children and young people with life-limiting
conditions is an active and total approach to care, embracing physical,
emotional, social and spiritual elements. It focuses on enhancement of
quality of life for the child and support for the family and includes the
management of distressing symptoms, provision of respite, and care through
death and bereavement.
Timing of the Test
All newborn infants are screened regardless of gestational age, weight, feeding or health status between 36 and 72 hours of life.
Palliated infants: A sample should still be obtained on palliated infants. This may help to exclude diseases which may have implications for the family in future pregnancies. If the sample is taken after the infant dies, write in red on the screening card that it is a “post-mortem” or “peri-mortem” sample as there is a marked difference in the metabolic profile between a live and a deceased infant.
Blood Sampling Table
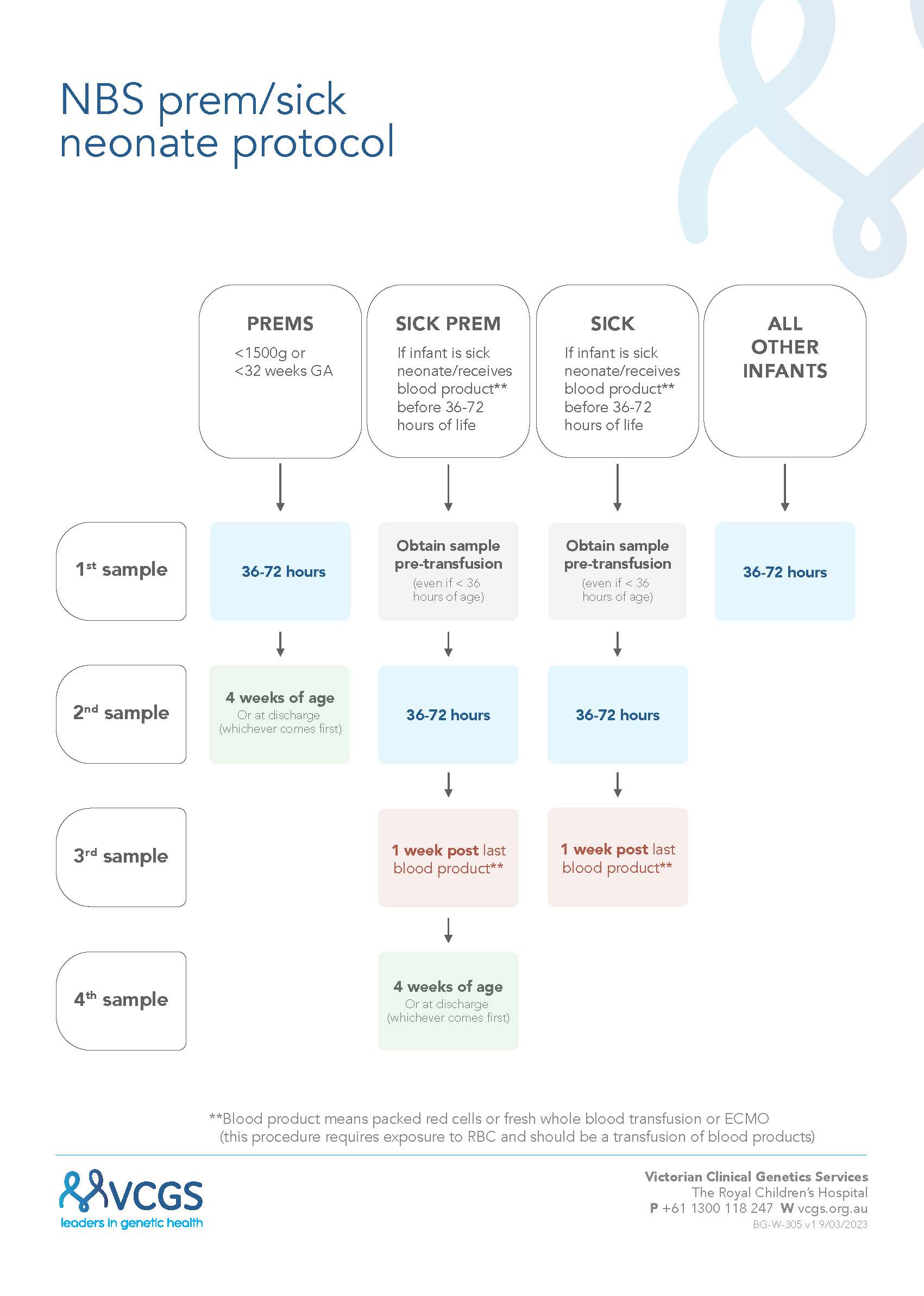
NBS flowchart - prem sick neonate v2.1 (vcgs.ninja)
****Blood product is now PRBC or whole blood transfusion or ECMO
If
recollection has been missed, collect sample as soon as this is
recognised.
If in doubt or you have any queries in regards to timing of screening, contact
the Newborn Bloodspot Screening Laboratory: Ext 16272
Responsibility Prior to Collection
Nursing staff on each shift are responsible for
checking that screening is performed or has been performed on infants in their
care.
- Nursing staff need to obtain written informed
consent from a parent/guardian prior to performing the test.
Note: if parent/guardian not available to provide
written consent - writing ‘verbal consent by phone’ is only acceptable with
written explanation as to why no parent/guardian signature obtainable. Written or verbal consent is only
applicable for the first card sample.
For subsequent samples, parents should know it has
been taken, but are not required to
sign each subsequent card.
- Parent information leaflet (Newborn Bloodspot
Screening – for the health of your baby) informs parents about the
screening program and should be given to the parents and discussed prior
to obtaining written consent. An interpreter may also be required if their
language not available on the website.
- The brochure, and the simplified version in 12
other languages is available on the Newborn Bloodspot Screening website.
The information leaflet can also be obtained from VCGS (Victoria Clinical
Genetic Service), 4th Floor East, Royal Children's Hospital.
| Ensure all details are filled out correctly on the newborn bloodspot screening card including: |
- Birth hospital and current hospital
- Date and time of birth
- Date and time of sample collection
- Gestation at birth
- Birth/Current weight
- If there has been a transfusion, list the date
- Tick box if baby is receiving TPN nutrition at time of sample
- If the baby is male or female gender
- Relevant clinical/family history –
refers to the metabolic conditions we are testing for and if the baby has
a past medical history of relevant conditions such as; meconium ileus,
severe jaundice or maternal hypothyroidism
- Collector’s name
|
Occasionally, stored newborn bloodspot screening cards get used for
research. Some examples are the development of new tests or determining normal
levels of a biomarker. This research is de-identified (i.e. no personal details
are released to the researchers) and must be approved by an ethics committee.
Parents can tick the “No Secondary Research Use” box if they do not wish their
child’s card to be available for such research
Refusal of Consent
If parents refuse to have the test the following steps should be followed:
Step 1: When parents or guardians are unsure or choose to decline screening, engage them in further discussion to determine their concerns and ensure they have not been misinformed. The storage and access to cards after screening are secondary issues. If their concerns are about storage and access to screening cards, reassure them they are free to opt out of research use and are able to request their baby’s card back after 2 years. It is vital that these issues do not deter parents from having their baby screened.
Step 2: Highlight the possible risks involved in not screening:
- It may lead to a delay in diagnosis and treatment of a serious medical condition.
- A delay in treatment could impair normal development and in rare cases, the condition may cause death.
- There are no alternatives to screening; by the time symptoms appear, development may already be affected.
Step 3: Offer to refer parents to a paediatrician or a newborn bloodspot screening counsellor, who will be able to further discuss any concerns. A newborn bloodspot screening counsellor can be contacted on 1300 118 247.
Step 4: If after discussion parents wish to decline screening, this decision must be respected. A parent is required to sign a written "decline of screening" form, indicating they understand the potential risks - this should be kept in the baby’s medical file, along with a description of the discussion that took place. Please ensure refusal is also documented in the baby’s maternal and child health record book.
You must also advise the family to seek medical attention if their baby is unwell and to tell the health-care provider the baby has not had newborn screening.
Step 5: Fill in a screening card as normal and have a parent sign that they are declining screening. Send this to the laboratory.
Sampling
| Collection |
|
| Procedural Pain
Management |
|
| Arterial Sample |
- For
arterial line sampling, ensure that the sample does not contain heparinised fluid. A discard of 2-3mls is recommended before using a new syringe to take the sample.
|
| Venous Sample |
- For venous sampling, refer to the blood sampling section of the RCH Policy
Central Venous Access Device Management. Ensure that the sample does not contain heparinised fluid. A discard of 2-3mls is recommended before using a new syringe to take the sample.
- A venous sample can also be collected via peripheral venous access. Refer to
Intravenous Access – Peripheral for sampling technique.
|
| Capillary Sample | |
Documentation
Record date and time of sample taken in:
| Electronic Medical
Record (EMR) |
Flowsheets > Primary Assessment > Newborn Bloodspot Screening (NBS).
|
| Child Health Record
book (Green book) |
In the ‘Birth Details’ section. |
Sending Samples
- Samples should be
airdried at the bedside for 4 hours
- Samples should then
be placed in a paper envelope and sent immediately after drying directly to VCGS Specimen Reception or to RCH
Specimen Reception (both via the pneumatic tube)
- VCGS Specimen
Reception – pneumatic tube #4 (send
to this station between 0700-1700 only)
- RCH Specimen
Reception – pneumatic tube #2 (send
to this station at all other times)
Special Considerations
For more information about what happens after
screening, please refer for the Newborn
Bloodspot Screening Information for Parents brochure.
Links
Other Resources
RCH Newborn Screening Clinical Nurse Consultants
Noelle Giordano, Tabitha Hole, Mia Normoyle
Phone: (03) 9345 6244 or (03) 9345 6062
Newborn Bloodspot Screening Laboratory
Victorian Clinical Genetic Services
Royal Children's Hospital, Parkville 3052
Tel: (03) 8341 6272 Fax: (03) 8341 6339
Email:
screeninglab@vcgs.org.au
Newborn Bloodspot Screening Nurse: Email:
sally.morrissy@vcgs.org.au
Phone: (03) 8341 6460
Evidence Table
Newborn Screening Evidence Table
Please remember to
read the disclaimer.
The development of this nursing guideline was coordinated by Lauren Cross, Registered Nurse/Midwife, Butterfly Ward, and approved by the Nursing Clinical Effectiveness Committee. Updated January 2023.