The paediatric clinical practice guidelines (CPGs) are point of care guidelines, developed to assist clinicians, particularly more junior clinicians, with decisions about appropriate health care for children and young people.
The CPGs are designed to provide concise practical advice regarding assessment and management of common and/or serious paediatric conditions. They are developed by a multidisciplinary team of practising clinicians, based on the available evidence and consensus.
The CPG Development Group
The CPG Development Group is made up of 2 part-time CPG Consultants, up to 6 part-time CPG Fellows (based in NSW, Qld and Vic) and part-time administration. This group manages CPG Committee meetings, and oversees development, publication and maintenance of CPGs hosted on the RCH website and the RCH CPG App. Email
cpg@rch.org.au for assistance.
The CPG Committee
The CPG Committee is made up of a broad group of clinicians from General Paediatrics, Emergency Medicine and General Practice, including doctors (consultants, trainees and junior medical staff), nurses and allied health practitioners from Victoria, New South Wales, Queensland, South Australia and Western Australia, with other states and territories anticipated to join in the coming year.
Each of the states has appointed its representatives to assist with adopting and adapting local jurisdictional practices and protocols for the development of the CPGs, ensuring relevance nationally for all CPG users.
Each CPG topic is allocated to a CPG Fellow who manages and
coordinates the end-to-end development/updating process. CPGs are written and
reviewed by Committee members; drafts are circulated for comment and then
discussed in detail at a committee meeting, held online via video link from 3-4pm (AEST) on the 1st Thursday and 3rd Wednesday of every month.
The Paediatric Improvement Collaborative (PIC)
CPGs have historically been produced by each state (and even individual hospitals) in Australia to support clinical practice. However, the production and maintenance of CPGs is a costly and time-consuming process. To better harness efforts, reduce variation in care, avoid duplication of work and reduce costs, the PIC was formed in July 2018 between Queensland Health Clinical Excellence Queensland (CEQ), the NSW Agency for Clinical Innovation (ACI), Safer Care Victoria (SCV) and the RCH. The initial aim was to adapt a number of Victorian paediatric CPGs to be appropriate for use in Queensland, New South Wales and Victoria. Since initial establishment, other states and territories have expressed interest in being involved and, in 2023, South Australia's Women's and Children's Health Network became a PIC partner. In the coming year it is planned to expand the PIC to include all states and territories, thereby becoming a national collaborative.
The PIC Steering Committee, with representation of all partners, has approved a CPG development and endorsement process, and provides governance to ensure that the process for development and revision of CPGs is robust and responsive.
CPG development process
The development of new CPG topics and the review cycle for existing CPGs is generally determined by the CPG Development Group, with input on priorities from the PIC Steering Committee. Topics/reviews may also be requested/suggested by State/Territory Health Departments and other organisations.
This flowchart describes the process:
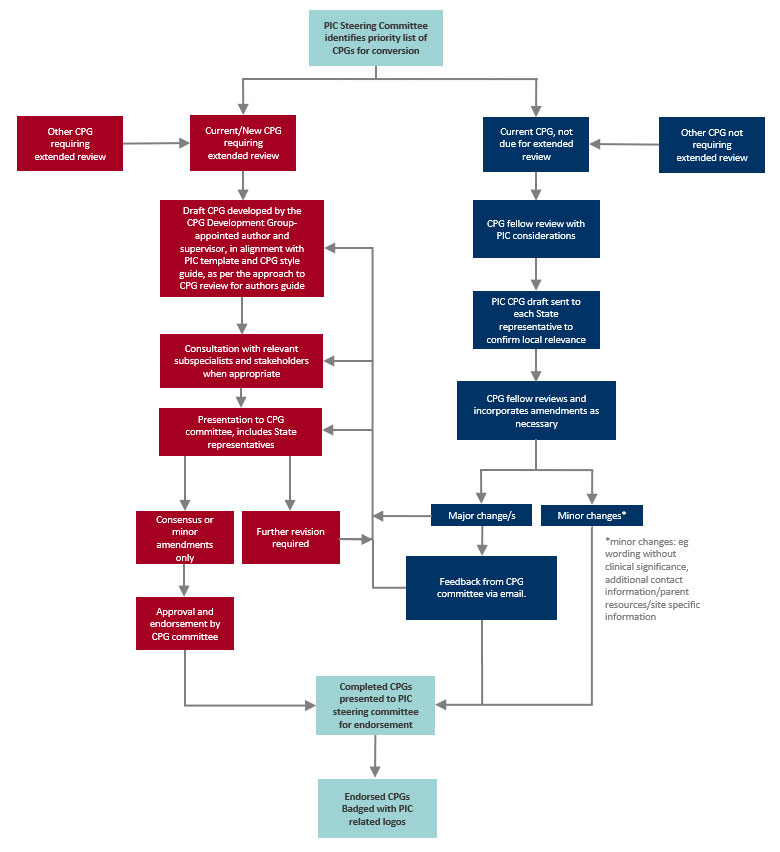
CPGs are reviewed regularly to ensure that they remain up to date; this is by one of two processes:
Extended review: Committee members and other clinicians undertake a detailed review of a current CPG or develop a new CPG after discussion with the CPG development group. Topics are chosen based on the authors’ areas of interest and priorities determined by the PIC.
Streamlined review: The CPG development group undertakes regular structured reviews considering current evidence, CPG content and formatting, to ensure all CPGs remain up to date. Subspecialists or relevant stakeholders may be contacted during the process for specific questions. Interstate input is also sought as part of this review. If significant changes are required, the CPG committee is consulted.
Other Resources
- CPG Development information for authors, supervisors and reviewers
- The clinical practice guideline page provides access to a number of guideline types, including some developed specifically for use at the RCH. The guideline index includes links to the RCH Nursing Clinical Guidelines, these will have a Clinical Guidelines (Nursing) banner at the top of each guideline.
Last updated March 2024