Note: This guideline is currently under review.
Introduction
Aim
Definition of Terms
Assessment
Management
Non-pharmacological
pain management
Procedural pain management & acute pain management
Post-operative and critically unwell pain management
Morphine
Paracetamol
Clonidine
Fentanyl
Hydromorphone
Dexmedetomidine
Opioid Conversion (IV)
Conversion of IV Morphine to Oral Morphine
Weaning Opioids (IV & Oral)
Weaning IV Dexmedetomidine
Weaning Oral Morphine
Weaning Clonidine
Special Considerations
Companion Documents
Links
Evidence Table
References
Introduction
Pain management in the acutely unwell neonate and/or post-operative period is not only humane; it is essential to minimise the endocrine, metabolic and neurologic responses to pain. It has been shown to significantly improve recovery time and healing and can prevent the development of chronic pain. However, exposure to opioids in the absence of pain may adversely impact the developing brain and neurodevelopmental outcomes. Judicious use of opioids in neonates is imperative and following a pain management algorithm has been shown to be effective in providing adequate pain management while minimising opioid exposure.
This guideline provides NICU clinicians with suggested pain and sedation management in the acutely unwell and/or post-operative neonate as a guide to individualise care. Each infant will respond to both pain and medications differently, clinical judgement, pain scores, gestational age, underlying diagnosis, previous exposure to opioids/sedatives and type of surgical procedure need to be considered in managing post-operative pain and sedation.
Aim
Pain management strategies will be focused on reducing (or minimising) pain during procedures and managing pain in critically unwell and/or post-operative neonates in the NICU.
Neonates being cared for in other wards/departments should refer to their local departmental guidelines and/or consult with the RCH Child Pain Management Service (CPMS) where appropriate.
Definition of Terms
| Term
|
Definition |
|
Analgesic
|
A medication where the primary indication is pain relief. |
|
Anxiolytic
|
A medication that relieves anxiety. |
|
Containment
|
A technique used to position the neonate to promote a sense of control and security for the neonate. Use two hands to hold the baby securely (i.e. one hand on the baby’s head and one on their feet). Positioning the neonate, appropriate to their gestational
maturation, supporting limbs/ trunk and taking care with any attached lines or equipment (i.e. supine or side lying). Rolls or position aids (or nests) can also be used. |
|
Facilitated tucking
|
A similar technique to containment which involves positioning the neonate to promote a sense of control and security. Hold the neonate so that their limbs are near their trunk. The neonate is held side lying in a flexed position. |
|
Iatrogenic Opioid Withdrawal
|
Physical symptoms (such as, tremors, sweating and irritability) which occur following the prolonged use of opioids and benzodiazepines during the intensive care unit stay. |
|
Neonatal Abstinence Syndrome (NAS)
|
A group of symptoms a neonate experiences from certain medications they are exposed to in utero before birth. NAS is most often caused when a woman takes opioids during pregnancy. |
|
Nesting
|
A positioning aid or roll that is placed around the neonate to help contain them and make them feel safe and secure by imitating a womb-like environment. It also helps keeps the neonate’s limbs in alignment when they cannot be wrapped or swaddled.
|
|
Non-Nutritive Sucking
|
Refers to the use of a dummy to promote sucking without breast milk or infant formula. |
|
Non-Pharmacologic Interventions
|
The use of strategies that use techniques to reduce pain other than medications, such as swaddling, non-nutritive sucking and positioning for pain relief. |
|
Opioid
|
Refers to natural, semi-synthetic and synthetic drugs that relieve pain by binding to opioid receptors in the nervous system e.g. morphine, hydromorphone. |
| Pain
|
An unpleasant sensory and emotional experience associated with, or resembling that associated with, actual or potential tissue damage (ISAP, 2020) |
|
Pharmacological
strategies
|
The use of drugs for pain relief. |
|
Sedative
|
A drug tending to calm, moderate, or tranquilize nervousness or excitement. |
|
Skin to
skin (Kangaroo care)
|
Nursing of the neonate on the bare skin of their mother or father, upright at a 40-60 degree angle and covered by parent’s shirt/gown, with an additional blanket as required. |
|
Swaddling
|
Neonates can be wrapped in a cloth or blanket, with their arms and legs tucked in, to make them feel secure. |
|
Tolerance
|
A process characterized by decreasing effects of a drug at its previous dose or the need for a higher dose of drug to maintain an effect. |
|
Weaning
analgesia or sedation
|
Titrating long-term pain relief and sedation at an slow and appropriate rate top avoid signs and symptoms of iatrogenic opioid withdrawal. |
Assessment
All neonates receiving pharmacological pain relief should have continuous cardiorespiratory monitoring insitu with hourly (minimum) observations documented. Sedation nearly always precedes respiratory depression, therefore this is an important observation in non-intubated neonates (Management of the paediatric patient receiving opioids).
The RCH NICU uses the modified Pain Assessment Tool (mPAT) to assess pain in neonates. The frequency of pain assessment depends on how many hours post-operation a neonate is and how long they have been on opioids (Neonatal Pain Assessment Nursing Guideline).
It is important to consider the procedure or surgery that the infant is going to and/or has experienced when assessing pain. Infants vary in their experience of and response to pain. The below list and table are guides to painful procedures and surgeries that frequently occur in the NICU.
Painful procedures include:
- Insertion and removal of chest drains
- Heel lance
- Intramuscular injection
- Subcutaneous injection
- Venepuncture
- Intravenous/Intra-arterial line insertion
- Central line insertion
- Lumbar Puncture
- Urinary catheter insertion
- Nasogastric tube insertion
- Single prong/Nasopharyngeal tube insertion
Table 1: The categorisation of pain associated with procedures/surgery.
|
Mild Pain
|
Moderate
Pain
|
Severe
Pain
|
- Bronchoscopy
- Laparoscopic surgeries
- Local stoma reversal/closure
- Inguinal hernia repair
- Ventricular shunt insertion
- Laser eye surgery
- Intravitreal Avastin
|
- Chest drain insertion
- Abdominal drain insertion
- Tracheostomy/critical airway procedure
- Uncomplicated Gastroschisis repair
- Gastroschisis silo formation
|
- Congenital diaphragmatic hernia (CDH) repair
- Oesophageal atresia and or/ tracheoesophageal repair
- Thoracotomy
- Laparotomy
- Operative Necrotising Enterocolitis
- Gastroschisis or omphalocele closure under tension
|
** Please consider the difficulty of the procedure/surgery and note that infants may experience and express pain differently. If an infant’s pain expression is more than expected, consider other factors for example, limb fractures, medical device placement or line
extravasations.
Long-term analgesic and sedative requirements:
For neonates who are ventilated, sedated, and requiring long-term analgesics and sedatives, it is important to consider the goals of care for each neonate, each day. While every neonate receives a sedation score (UMSS – University of Michigan Sedation Score) from 4 – unrousable to 0 – awake and alert, every shift as part of the ‘primary assessment’ flowsheet on EMR, it is important for the multidisciplinary team to consider the acceptable level of sedation for each neonate each day while they are in the acute phase of their postoperative journey. This determines if /when it is appropriate to start weaning analgesics and sedation to reduce the occurrence of opioid tolerance and iatrogenic opioid withdrawal.
Management
The four basic principles underpinning pain management are:
1. Regular pain assessment and consideration of goals of patient care
2. Appropriate and timely medication administration
3. The use of multi-modal pain management techniques
4. Avoidance and careful management of side effects
Non-pharmacological
pain management
All neonates should receive physical/psychological developmentally appropriate strategies during all painful procedures. Non-pharmacological strategies should be used in addition to pharmacological strategies. The neonate’s parents and families should be involved in these strategies during painful procedures, when
feasible.
1. Skin to skin contact (Kangaroo care)
2. Breastfeeding (dependent on mother’s intention & neonate’s ability to breastfeed)
3. Non-nutritive sucking (Consent from parents to use a dummy/pacifier required)
4. Positioning and containment
5. Nesting and/or facilitated tucking)
6. Swaddling
7. Reduction of stimuli (light and sound)
8. Minimal handling
9. Voice or singing and music therapy
10. Cuddle, positive touch or movement
It is encouraged to use more than one neurodevelopmental strategy simultaneously, for example, non-nutritive sucking with swaddling and reduction of stimuli. More information on neurodevelopmental care strategies can be found on in the
Neurodevelopmental Care Learning Hero Package.
It is important to engage parents in neurodevelopmental care strategies during non-urgent ‘minor’ painful procedures, such as heel lances and venepuncture, as it enhances the benefits of these techniques for the neonate to have their parents and family present delivering this care (
COCOON Learning Package).
Procedural
pain management & acute pain management
Oral sucrose is a safe and effective mild analgesic, which is effective in decreasing short-term pain and distress during minor procedures. (
Nursing Guideline: Sucrose (oral) for procedural pain management in infants)
Suggested opioid bolus ranges*:
| Medication |
Ventilated |
Self-ventilating |
| Morphine |
25 - 100mcg/kg |
10 - 25mcg/kg |
| Fentanyl |
1 - 5 mcg/kg |
1 - 2mcg/kg |
| Hydromorphone |
5 - 10 mcg/kg |
|
*Higher doses may be required in opioid-tolerant patients and may be used with consultant approval
** Consider starting with smaller doses for opioid naïve patients.
Also consider chloral hydrate, clonidine and dexmedetomidine for procedures to provide sedation for agitated and distressed neonates. Remember to administer adequate pain relief in addition to sedatives. If no IV access, consider oral morphine and time to effectiveness prior to procedure.
Post-operative
and critically unwell pain management
Flowchart 1: Pain management in the Postoperative or Critically Unwell Neonate:
.jpg)
*Most neonates will have their pain well managed with morphine, paracetamol and clonidine. Midazolam use should be minimised due to its potentially harmful effects on neurodevelopment. If the complexity of the neonate’s condition requires midazolam, this should only be used with
consultant approval.
It is important to continually assess the neonates mPAT scores and the goals of care for the neonate (i.e. the appropriate level of sedation) to consider weaning and de-escalating pain treatment to avoid tolerance and iatrogenic opioid withdrawal. The neonate’s goals of care should be clear to
the multidisciplinary team and the parents/families at the bedside to allow for appropriate patient care and advocacy.
Morphine: Please see RCH Medication Guideline: RCH Morphine Continuous infusion
Paracetamol:
- Paracetamol to be administered regularly in the first 48 hours post-operative or after acute deterioration then consider PRN.
- Dosing as per the neonate’s gestational age in the
Neonatal Formulary.
Clonidine:
For more information, please see the RCH departmental guideline: Butterfly clonidine medication guideline.
Fentanyl:
Consider converting to fentanyl if morphine infusion reaches maximum dosage and remains ineffective.
| Syringe concentration |
0.1mg/kg of fentanyl in 25mL of compatible diluent |
| 1mL/h equal |
4mcg/kg/hr |
| Infusion range |
1-5mcg/kg/hr |
Hydromorphone:
Consider converting to hydromorphone and refer to CPMS if fentanyl infusion reaches maximum dosage and remains ineffective. Hydromorphone requires DUC approval.
| Syringe concentration |
0.25mg/kg of hydromorphone in 25mL of compatible diluent |
| 1mL/h equal |
10mcg/kg/hr |
| Infusion range |
3-24mcg/kg/hr |
Dexmedetomidine:
Consider converting to dexmedetomidine if clonidine infusion reaches maximum dosage and remains ineffective. To reduce the risk of withdrawal, the use of dexmedetomidine is limited to 3-7 days. Dexmedetomidine requires DUC approval.
- Dexmedetomidine has standard syringe concentrations and is not weight based.
- NOT FOR IV BOLUS ADMINISTRATION.
| Syringe concentration |
200 mcg of dexmedetomidine in 50mL of 0.9% NaCl
(4 mcg/mL) |
| Commencement rate |
0.1 mcg/kg/hr |
| Infusion range |
0.1-0.6 mcg/kg/hr
Increased up to 1.2 mcg/kg/hr with consultant approval |
| Consider loading dose at infusion commencement |
0.5 – 1 mcg/kg over 15 minutes |
Dexmedetomidine may also be used as a weaning agent to facilitate the reduction of opioids to aid with a more timely extubation in chronic, long-term patients, as dexmedetomidine does not effect respiratory drive.
Opioid
Conversion (IV)
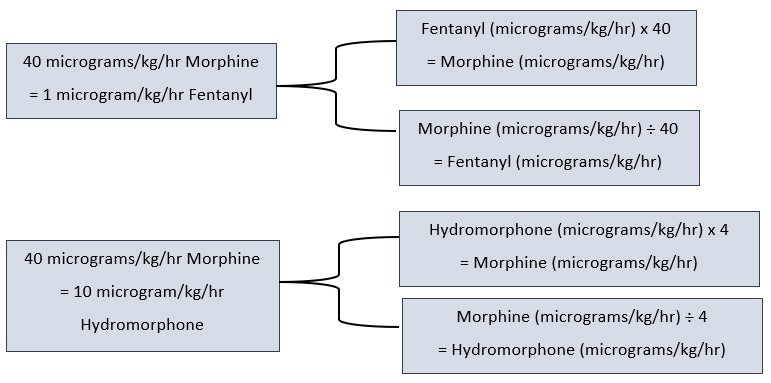
- If switching opioids due to adverse effects, consider reducing the total daily dose after conversion by 25% to allow for cross tolerance.
- If cycling opioid for inadequate pain management, then do not reduce the converted amount of opioid for cross-tolerance.
Conversion
of IV Morphine to Oral Morphine

![]()
Weaning
Opioids (IV & Oral)
Please see RCH Departmental Guideline: Weaning Analgesia and Sedation on NICU
As the neonates mPAT scores reduce and the neonate is recovering from their acute deterioration and/or surgery, analgesia and sedation should be weaned as aligned with the neonate’s long term care goals. This should be clearly communicated with the multidisciplinary team and parents/families
and documented in the progress notes in EMR.
The neonate should have regular neonatal abstinence scores (or Finnegan’s scores) as per the departmental guideline and resources (
Neonatal abstinence scoring). Ensure the neonate has clonidine and/or paracetamol prescribed while weaning opioid analgesia. If prescribed regular clonidine, this should be weaned last.
| Duration
of opioid use |
Wean rate
|
| Less than
1 day for non-postoperative babies |
Reduce rate by 50% of highest dose and cease when clinically appropriate. |
| Less than
1 day for postoperative babies |
Do not wean for first 12 hours post-operatively unless medically indicated and following discussion with treating medical team (weaning may be appropriate for some surgical procedures). After this time, reduce rate by 10–20% of the highest dose every 4–6 hours to maintain mPAT scores
<10.
|
![]() 1–4 days 1–4 days |
Reduce rate by 10–20% of the highest dose every 4–6 hours to maintain mPAT scores
<10. |
| Equal to
or greater than 5 days (commence NAS on day 5) |
Reduce rate by 10–20% per day of the highest dose until ceased or changed to enteral therapy as per consultant. |
Weaning IV
Dexmedetomidine
| Duration of dexmedetomidine use |
Wean rate |
| Less than 24 hours |
Infusion can be ceased without weaning. |
| 24–72 hours |
Halve the infusion. If haemodynamically stable after
2 hours, wean by 0.1 micrograms/kg/hr every 4-6 hours. |
| Greater than 72 hours |
Wean by 0.1 micrograms/kg/hr every 12–24 hours until ceased. |
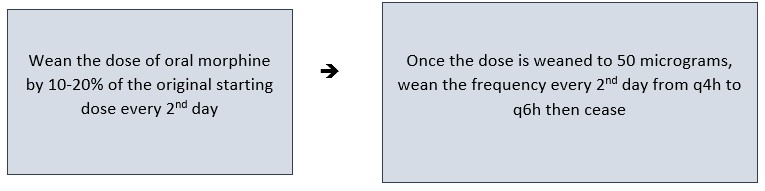
Weaning
Oral Morphine
Iatrogenic opioid withdrawal is different to neonatal abstinence syndrome. The following information pertains to weaning from opioids administered during a neonates NICU journey. The regime to wean oral morphine for a neonate with neonatal abstinence syndrome is different. For information on neonatal abstinence
syndrome, please refer to the Butterfly departmental guideline
here.
When weaning from oral morphine reduce the dosage by 10–20% of the dose that the patient was receiving when weaning commenced e.g. if the starting dose was 500 micrograms when weaning commenced wean by 50 – 100 microgram increments (the same amount each time rather than weaning each individual dose 10-20%).
Weaning Clonidine
Clonidine requires weaning if used for more than 5 days (after the opioid/ benzodiazepine weaning is complete) to avoid side effects, such as rebound hypertension. The clonidine dose should be weaned by about 50% each day for 2 to 3 days (reflecting an average
half-life of 17 hours in neonates) before ceasing the drug. Watch for tachycardia, hypertension, sweating, agitation, but remember these may also be opioid withdrawal symptoms.
Special Considerations
Renal and
Liver Disease Dosing Adjustment Recommendations
| |
Renal
Disease Implications |
Liver
Disease Implications |
| Morphine |
Avoid in renal disease patients and patients requiring dialysis |
Reduce dose by 50% |
| Fentanyl |
No clinically significant accumulation |
None |
| Hydromorphone |
Reduce dose by 25% |
Limited data |
| Clonidine |
Dose reduction may be required as side-effects of bradycardia and hypotension may be more likely |
None |
Companion Documents
- Butterfly COCOON Website
- COCOON Learning Package
- Neonatal Formulary
- Nursing Guideline: Sucrose (oral) for procedural pain management in infants
- Neurodevelopmental Care Learning Hero Package
- Neonatal Pain Assessment Nursing Guideline
- Neonatal Pain Assessment Learning Hero Package
Links
- Safer Care Victoria: Intensive care pain, agitation and delirium standardised assessment and monitoring
- Safer Care Victoria: Developmental care for neonates
- Safer Care Victoria: Sucrose for procedural pain in neonates
- The Australian and New Zealand College of Anaesthetists: Acute Pain Management Scientific Evidence 5th edition Volume 1 and Volume 2 (Paediatrics)
- Safer Care Victoria: Substance Use During Pregnancy – Care of the Mother and Newborn
- Royal
Prince Alfred Hospital: Australasian Neonatal Medicines Formulary
Evidence Table
The evidence table for this guideline can be viewed here.
References
- Grabski DF, Vavolizza RD, Lepore S, Levin D, Rasmussen SK, Swanson JR, et al. A Quality Improvement Intervention To Reduce Postoperative Opiate Use in Neonates. Pediatrics. 2020;146(6):1-9. doi: 10.1542/peds.2019-3861. PubMed PMID: 147956455. Language: English. Entry Date: 20210110. Revision Date: 20210111.
Publication Type: Article.
- Zhu A, Benzon HA, Anderson TA. Evidence for the Efficacy of Systemic Opioid-Sparing Analgesics in Pediatric Surgical Populations: A Systematic Review. Anesthesia And Analgesia. 2017;125(5):1569-87. doi: 10.1213/ANE.0000000000002434. PubMed PMID: 29049110.
- Hammer GB, Maxwell LG, Taicher BM, Visoiu M, Cooper DS, Szmuk P, et al. Randomized Population Pharmacokinetic Analysis and Safety of Intravenous Acetaminophen for Acute Postoperative Pain in Neonates and Infants. Journal of Clinical Pharmacology. 2020;60(1):16-27. doi: 10.1002/jcph.1508. PubMed PMID: 141075977.
Language: English. Entry Date: 20200110. Revision Date: 20200110. Publication Type: Article. Journal Subset: Biomedical.
- Zeilmaker-Roest GA, van Rosmalen J, van Dijk M, Koomen E, Jansen NJG, Kneyber MCJ, et al. Intravenous morphine versus intravenous paracetamol after cardiac surgery in neonates and infants: a study protocol for a randomized controlled trial. Trials. 2018;19(1):N.PAG-N.PAG. doi: 10.1186/s13063-018-2705-5.
- Allegaert K, van den Anker JN. Perinatal and neonatal use of paracetamol for pain relief. Seminars In Fetal & Neonatal Medicine. 2017;22(5):308-13. doi: 10.1016/j.siny.2017.07.006. PubMed PMID: 28720398.
- Baarslag MA, Allegaert K, Van Den Anker JN, Knibbe CAJ, Van Dijk M, Simons SHP, et al. Paracetamol and morphine for infant and neonatal pain; still a long way to go? Expert Review Of Clinical Pharmacology. 2017;10(1):111-26. doi: 10.1080/17512433.2017.1254040. PubMed PMID: 27785937.
- Härmä A, Aikio O, Hallman M, Saarela T. Intravenous Paracetamol Decreases Requirements of Morphine in Very Preterm Infants. The Journal Of Pediatrics. 2016;168:36-40. doi: 10.1016/j.jpeds.2015.08.003. PubMed PMID: 26323200.
- Schiller RM, Allegaert K, Hunfeld M, van den Bosch GE, van den Anker J, Tibboel D. Analgesics and Sedatives in Critically Ill Newborns and Infants: The Impact on Long-Term Neurodevelopment. Journal Of Clinical Pharmacology. 2018;58 Suppl 10:S140-S50. doi: 10.1002/jcph.1139. PubMed PMID: 30248203.
- Moriarty C, Carroll W. Paracetamol: pharmacology, prescribing and controversies. Archives Of Disease In Childhood Education And Practice Edition. 2016;101(6):331-4. doi: 10.1136/archdischild-2014-307287. PubMed PMID: 27206455.
- Roofthooft DWE, Simons SHP, van Lingen RA, Tibboel D, van den Anker JN, Reiss IKH, et al. Randomized Controlled Trial Comparing Different Single Doses of Intravenous Paracetamol for Placement of Peripherally Inserted Central Catheters in Preterm Infants. Neonatology. 2017;112(2):150-8. doi: 10.1159/000468975. PubMed PMID:
28558384.
- Kabataş EU, Dursun A, Beken S, Dilli D, Zenciroğlu A, Okumuş N. Efficacy of Single Dose Oral Paracetamol in Reducing Pain During Examination for Retinopathy of Prematurity: A Blinded Randomized Controlled Trial. Indian Journal Of Pediatrics. 2016;83(1):22-6. doi: 10.1007/s12098-015-1765-8. PubMed PMID: 25947264.
- Smits A, van den Anker JN, Allegaert K. Clinical pharmacology of analgosedatives in neonates: ways to improve their safe and effective use. The Journal Of Pharmacy And Pharmacology. 2017;69(4):350-60. doi: 10.1111/jphp.12599. PubMed PMID: 27364566.
- Ohlsson A, Shah PS. Paracetamol (acetaminophen) for prevention or treatment of pain in newborns. The Cochrane Database Of Systematic Reviews. 2016;10:CD011219. doi: 10.1002/14651858.CD011219.pub4. PubMed PMID: 27716943.
- Baarslag MA, Ista E, de Leeuw T, van Rosmalen J, Tibboel D, van Dijk M, et al. Clinically effective implementation of intravenous paracetamol as primary analgesia after major surgery in neonates and young infants. Archives Of Disease In Childhood. 2018;103(12):1168-9. doi: 10.1136/archdischild-2018-315379.
PubMed PMID: 29991471.
- McPherson C, Ortinau CM, Vesoulis Z. Practical approaches to sedation and analgesia in the newborn. Journal of Perinatology. 2021;41(3):383. doi: 10.1038/s41372-020-00878-7. PubMed PMID: edssjs.2B8608C3.
- Abiramalatha T, Mathew SK, Mathew BS, Shabeer MP, Arulappan G, Kumar M, et al. Continuous infusion versus intermittent bolus doses of fentanyl for analgesia and sedation in neonates: an open-label randomised controlled trial. Archives Of Disease In Childhood Fetal And Neonatal Edition. 2019;104(4):F433-F9. doi:
10.1136/archdischild-2018-315345. PubMed PMID: 30322973.
- Välitalo PA, Krekels EH, van Dijk M, Simons S, Tibboel D, Knibbe CA. Morphine Pharmacodynamics in Mechanically Ventilated Preterm Neonates Undergoing Endotracheal Suctioning. CPT: Pharmacometrics & Systems Pharmacology. 2017;6(4):239-48. doi: 10.1002/psp4.12156. PubMed PMID: 28109060.
- Meesters NJ, van Dijk M, Knibbe CAJ, Keyzer-Dekker CMG, Tibboel D, Simons SHP. Infants Operated on for Necrotizing Enterocolitis: Towards Evidence-Based Pain Guidelines. Neonatology. 2016;110(3):190-7. doi: 10.1159/000445284. PubMed PMID: 27198526.
- Schuurmans J, Benders M, Lemmers P, van Bel F. Neonatal morphine in extremely and very preterm neonates: its effect on the developing brain - a review. The Journal Of Maternal-Fetal & Neonatal Medicine: The Official Journal Of The European Association Of Perinatal Medicine, The Federation Of Asia And Oceania Perinatal
Societies, The International Society Of Perinatal Obstetricians. 2015;28(2):222-8. doi: 10.3109/14767058.2014.908178. PubMed PMID: 24670240.
- Bellù R, Romantsik O, Nava C, Waal KA, Zanini R, Bruschettini M. Opioids for newborn infants receiving mechanical ventilation. Cochrane Database of Systematic Reviews. 2021(3). doi: 10.1002/14651858.CD013732.pub2. PubMed PMID: edschh.CD013732.
- Elkomy MH, Drover DR, Galinkin JL, Hammer GB, Glotzbach KL. Pharmacodynamic Analysis of Morphine Time-to-Remedication Events in Infants and Young Children After Congenital Heart Surgery. Clinical Pharmacokinetics. 2016;55(10):1217-26. doi: 10.1007/s40262-016-0398-z. PubMed PMID: 27098060.
- Rostas SE. Dexmedetomidine: A Solution to the Dilemma of Pain and Agitation in the Mechanically Ventilated Preterm Neonate? The Journal Of Perinatal & Neonatal Nursing. 2017;31(2):104-8. doi: 10.1097/JPN.0000000000000251. PubMed PMID: 28437301.
- Michel J, Hofbeck M, Peper A-K, Kumpf M, Neunhoeffer F. Evaluation of an updated sedation protocol to reduce benzodiazepines in a pediatric intensive care unit. 2020. p. 1-6.
- Parikh JM, Amolenda P, Rutledge J, Szabova A, Chidambaran V. An update on the safety of prescribing opioids in pediatrics. Expert Opinion On Drug Safety. 2019;18(2):127-43. doi: 10.1080/14740338.2019.1571037. PubMed PMID: 30650988.
- Fallah R, Habibian S, Noori-Shadkam M. Efficacy and Safety of Single Low Dose Intravenous Fentanyl in Pain Reduction of Lumbar Puncture in Near Term Neonates by A Randomized Clinical Trial. Iranian Journal of Child Neurology. 2016;10(2):60-6. PubMed PMID: 114486127. Language: English. Entry Date:
20180117. Revision Date: 20190213. Publication Type: Article.
- Zeller B, Giebe J. Opioid Analgesics for Sedation and Analgesia During Mechanical Ventilation. Neonatal Network: NN. 2015;34(2):113-6. doi: 10.1891/0730-0832.34.2.113. PubMed PMID: 26803092.
Please remember to read the disclaimer.
The development of this nursing guideline was coordinated by Bianca Devsam, Clinical Nurse Specialist, Butterfly Ward, Neonatal Intensive Care Unit, approved by the Nursing Clinical Effectiveness Committee. Published December 2021.