Introduction
Air or particles introduced into the patient circulation via venous access can have catastrophic consequences for patients who have communications between their arterial and venous circulations. Venous emboli that cross from the right side (venous circulation) to the left side of
the heart into the arterial circulation bypass filtering through pulmonary capillary diffusion into alveoli and therefore have greater proximity to the coronary and cerebral circulations. Hence, there is a greater need for filtering emboli for patients with mixed venous and arterial circulations. Using
0.2micron in-line filters can help prevent air, particle, and microbe introduction into the patient circulation via intravenous lines.
Aim
- To identify cardiac patients who are at an increased risk of systemic emboli from air or particles entrained into venous access
- To provide information on the management of in-line filters to minimise the risk of embolisation in these patients
- To demonstrate the correct position of in-line filters in venous access lines
Management
Cardiac patients who require filters
- In-line filters must be placed in all direct atrial lines.
- In-line filters must be placed in all arterial and all central and peripheral intravenous lines in the following circumstances:
- Pre-operative or unrepaired patients with a congenital heart defect with communications between their venous and arterial circulations.
- Pre and post-operative patients with single ventricle lesions.
Filters available
A 0.2micron filter filters particulate debris, microbial contaminants and their endotoxins and entrained air. Once the membrane of the filter is wet during priming, air is no longer able to enter the patient.
Two sizes are used at the Royal Children’s Hospital.
1. Pall Nanodyne®NEO Filter (Figure 1)
Priming volume of 0.4mL
Maximum flow rate of 110mL/hr
Should be used for neonates and infants
2. Pall Nanodyne ® ELD
Filter (Figure 2)
Priming volume of 2.6mL
Maximum flow rate of 1260-1380 mL/hr
Both filters need to be changed every 96 hours (4 days) to assure microbe and endotoxin retention.
Filters administering parenteral nutrition must be changed every 24 hours.
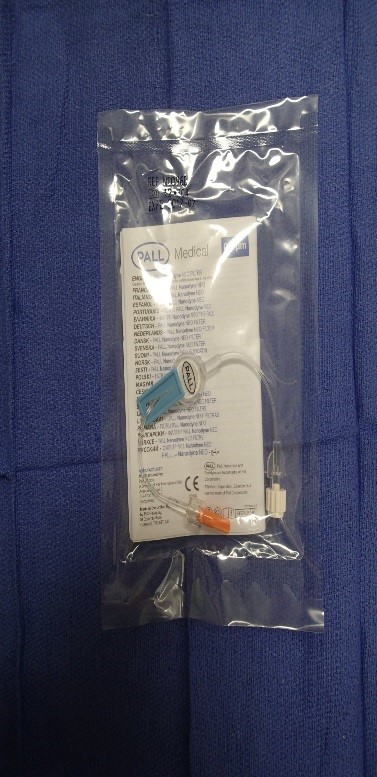
Figure 1. Pall Nanodyne ®NEO filter
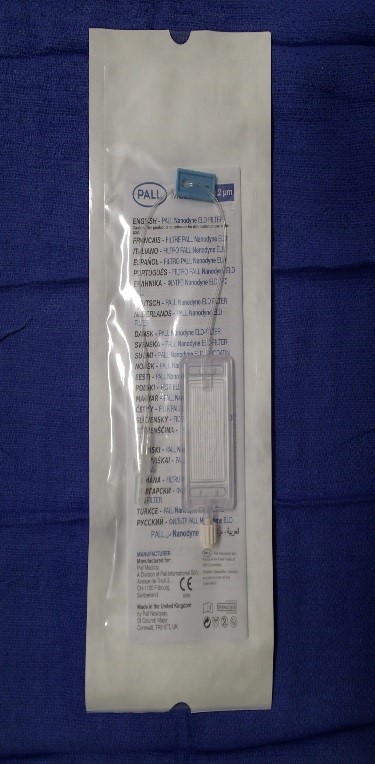
Figure 2. Pall Nanodyne ® ELD filter
Pall Nanodyne® NEO 0.2micron filters (Figure 1) can be used for Epoprostenolol (Prostacyclin) infusions. This filter is to be changed every 24 hours.
Placement and use of filters
Identify in advance the patients likely to require an in-line filter before their admission to the unit or arrival from theatre.
For postoperative cardiac patients requiring inotrope infusions, filters should be placed onto the intravenous lines by the anaesthetist at the commencement of the infusion in theatre. When this has not occurred, the infusion should be changed to a venous line with a filter as soon as the patient’s condition
allows.
Multiple infusions that are compatible for administration via one lumen and do not require bolus (e.g., inotropes line) can be infused through a shared filter where the filter should be attached to a needless adaptor as close as possible to the patient. See example in Figure 3.
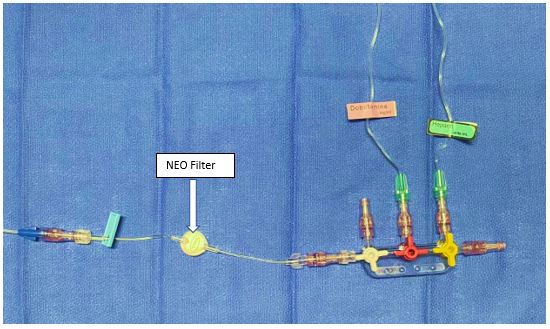
Figure 3. One in-line filter is required near the patient hub while multiple inotropes can be infused distally via three-way taps
Volume and maintenance CVAD lumen
In the event a fluid bolus must be administered, an in-line filter would impede quick and easy administration. Despite the risk of emboli, an in-line filter cannot be used in the venous line used for filling. Volume should be administered via a three-way tap or ‘traffic light’ system and may added to the CVAD maintenance
line if required (See Figure 4). Other drug ports and the maintenance lines that share a lumen with the filling line should have their own individual filters.
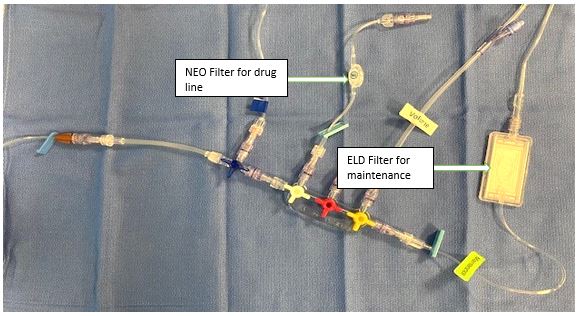
Figure 4. Set up of maintenance CVAD lumen (with volume line if required)
Invasive monitoring or direct atrial
lines in ICU
Link to
invasive haemodynamic cardiac monitoring guideline HERE
Place the filter between the syringe and transducer, so that it does not interfere with the pressure reading (see Figure 5). Filters may result in a slightly dampened waveform trace. Turning the three-way tap to temporarily isolate the transducer from the infusion may assist a more detailed waveform analysis.
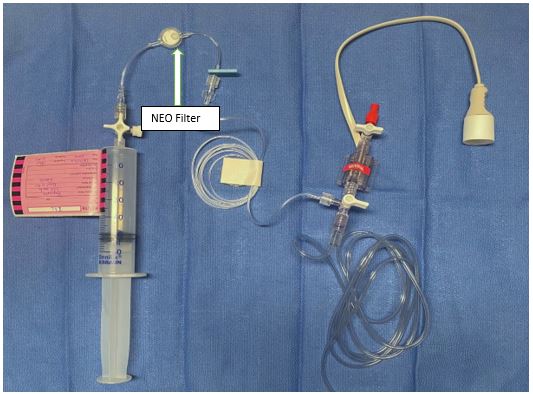
Figure 5. Set up of pressure monitoring lines in PICU
Medications that cannot be filtered
Most medications and fluids commonly administered to cardiac patients can be filtered through the 0.2 micron filter.
Medications with limited evidence that cannot be filtered through a 0.2micron filter include:
- Amphotericin
- Asparaginase
- Blood products
- Cyclosporin
- Lipid
- Octreotide
- Propofol
Substances that cannot be filtered should ideally be infused by themselves. In situations in which this is not possible, the unfiltered infusion should be attached as close to the patient as possible. All other compatible infusions should be infused through a filter.
In the event a patient requires a CT with contrast, ensure contrast is administered through a venous line without a filter as the filter will delay the delivery of contrast.
Troubleshooting
If you notice increasing pressure on your infusion pump(s) or occlusion alarms, this may be due to a blocked filter. A blocked filter does not mean the filter has failed. Rather it’s more likely the filter has succeeded in stopping debris from entering the patient’s
circulation. A VHIMS listing the fluids/drugs infusing through the filter and their infusing rates should be completed. This will add to the body of information about infusion (in)compatibilities.
Companion Documents
- RCH Departmental Guidelines
- RCH Policies and Procedures
Evidence Table
The
evidence table for this guideline can be viewed here.
Please remember to
read the disclaimer.
The revision of this nursing guideline was coordinated by Sathika Tilakaratne, RN, PICU, approved by the Nursing Clinical Effectiveness Committee. Published January 2023.