See also:
Poisoning – acute guidelines for initial management
Resuscitation
Relevant sections in this guideline
For 24-hour advice, contact the Poisons Information Centre 13 11 26
Intravenous paracetamol medication errors are not covered in these guidelines and must be discussed with Poisons Information Centre or a clinical toxicologist
Key points
- Suspect paracetamol poisoning in all deliberate adolescent self-poisonings and consider other co-ingestants
- Time to acetylcysteine is crucial in protecting the liver from significant toxicity. Stated timing and dose of paracetamol ingestion can be unreliable
- Check that the laboratory result matches the correct units on the nomogram (in mg/L or micromol/L)
- Management differs between immediate release, modified release and repeated supratherapeutic paracetamol ingestions
- Complicated ingestions eg longer-acting forms of paracetamol (modified release, eg Panadol Osteo) or repeated supratherapeutic ingestions should be discussed with Poisons Information Centre or a clinical toxicologist
Background
- Paracetamol is involved in a large proportion of accidental paediatric exposures and deliberate self-poisonings
- Hepatic failure or death is uncommon in treated individuals
- Acetylcysteine is a safe and effective antidote. “NAC” is not an approved abbreviation and acetylcysteine should be written in full
- Increased risk of liver injury is associated with delays (>8 hours post ingestion) in acetylcysteine administration
Children requiring assessment
- Any symptomatic child
- All children with deliberate self-poisoning
- Any child whose developmental age is inconsistent with accidental poisoning as non-accidental poisoning should be considered
- Ingestion of unknown quantity
- Acute ingestion of >200 mg/kg or >10 g (whichever is less)
- Repeated supratherapeutic ingestion
- >200 mg/kg or >10 g (whichever is less) over a single 24-hour period
- >300 mg/kg or >12 g (whichever is less) over a single 48-hour period
- Greater than a daily therapeutic dose per day for more than 48 hours in patients who also have abdominal pain, nausea or vomiting
Risk assessment
History
- Intentional overdose or accidental (consider the possibility of co-ingestions)
- Time of ingestion
- Dose: stated or likely dose taken
- Form: liquid, suppository, tablet
- Formulation: immediate or modified release tablet (important as this dictates management)
- If possible, determine the exact name and tablet strength
- Paracetamol is in many combination products
- Poisons Information Centre can assist with medication identification if specific product name is known
- Calculate the maximum possible dose per kg
Examination
- Many patients with significant paracetamol toxicity may not have signs or symptoms
- The main signs of paracetamol poisoning are hepatotoxicity which may take 48-72 hours to develop
- RUQ tenderness
- Hypotension
- Hypoglycaemia
- Acute kidney injury
- Encephalopathy
- Signs of fulminant hepatic failure and coagulopathy (may occur >72 hours)
Always check for Medicalert bracelet in any unconscious patient, or any other signs of underlying medical condition (fingerprick marks etc)
Paracetamol concentration nomogram
- Determines the need for acetylcysteine administration in acute immediate release and modified release preparations
- Should not be used for repeated supra-therapeutic ingestions
- Plot serum paracetamol concentration against time of ingestion on the nomogram
- Ensure that the units of measurement are the same as the scale used on the nomogram
- If the measured concentration is below the line, no treatment is required
- If the measured concentration is above the line, acetylcysteine treatment is needed
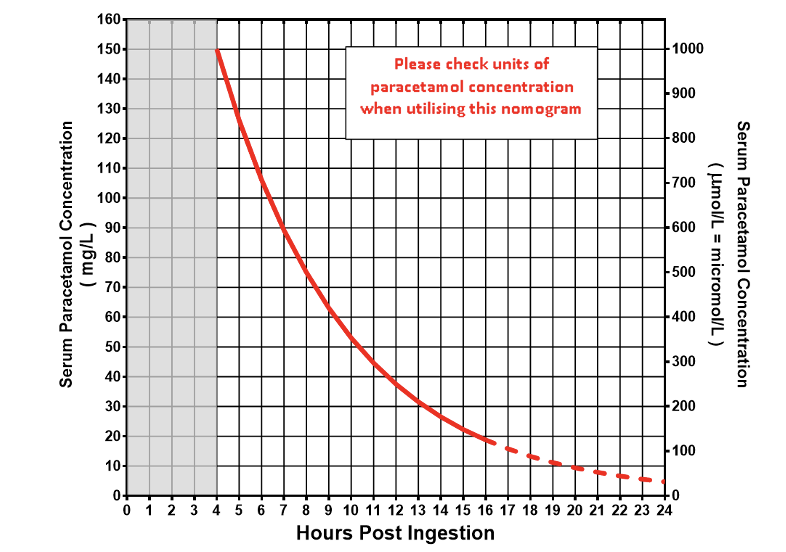
Paracetamol treatment nomogram (Rumack-Matthew nomogram)
Single dose, immediate release paracetamol ingestion
Investigations
- Serum paracetamol concentration and LFT taken at
- 4 hours (or as soon as possible after) post ingestion of tablets
- 2 hours post ingestion of a liquid preparation
- INR if more than 24 hours post ingestion or abnormal ALT
- Commence acetylcysteine immediately if
- Paracetamol concentration result is not likely to be available within 8 hours of a potentially toxic ingestion
- Child has symptoms of hepatic injury
Acute management
Decontamination
- Activated charcoal
- has a very limited role in treatment and should only be used in consultation with a toxicologist
- may be offered up to 2 hours post ingestion, if dose >200 mg/kg or >10 g
- Unlikely to be required in children under 6 years old as significant paracetamol overdose and hepatic injury is uncommon
Ongoing care and management
- See flowchart below, to be used in combination with nomogram and acetylcysteine infusion guide
Liquid paracetamol ingestion
In well children <6 years of age suspected of ingesting >200 mg/kg of liquid paracetamol, measure serum paracetamol concentration at 2-4 hours post-ingestion.
- Paracetamol concentration <150 mg/L (1000 micromol/L): acetylcysteine is not required
- Paracetamol concentration ≥150 mg/L: repeat concentration again at 4 hours post-ingestion. If the concentration is still ≥150 mg/L (1000 micromol/L), commence acetylcysteine infusion
For children presenting >4 hours post ingestion or ≥6 years of age, treat as per the acute ingestion management flowchart below.
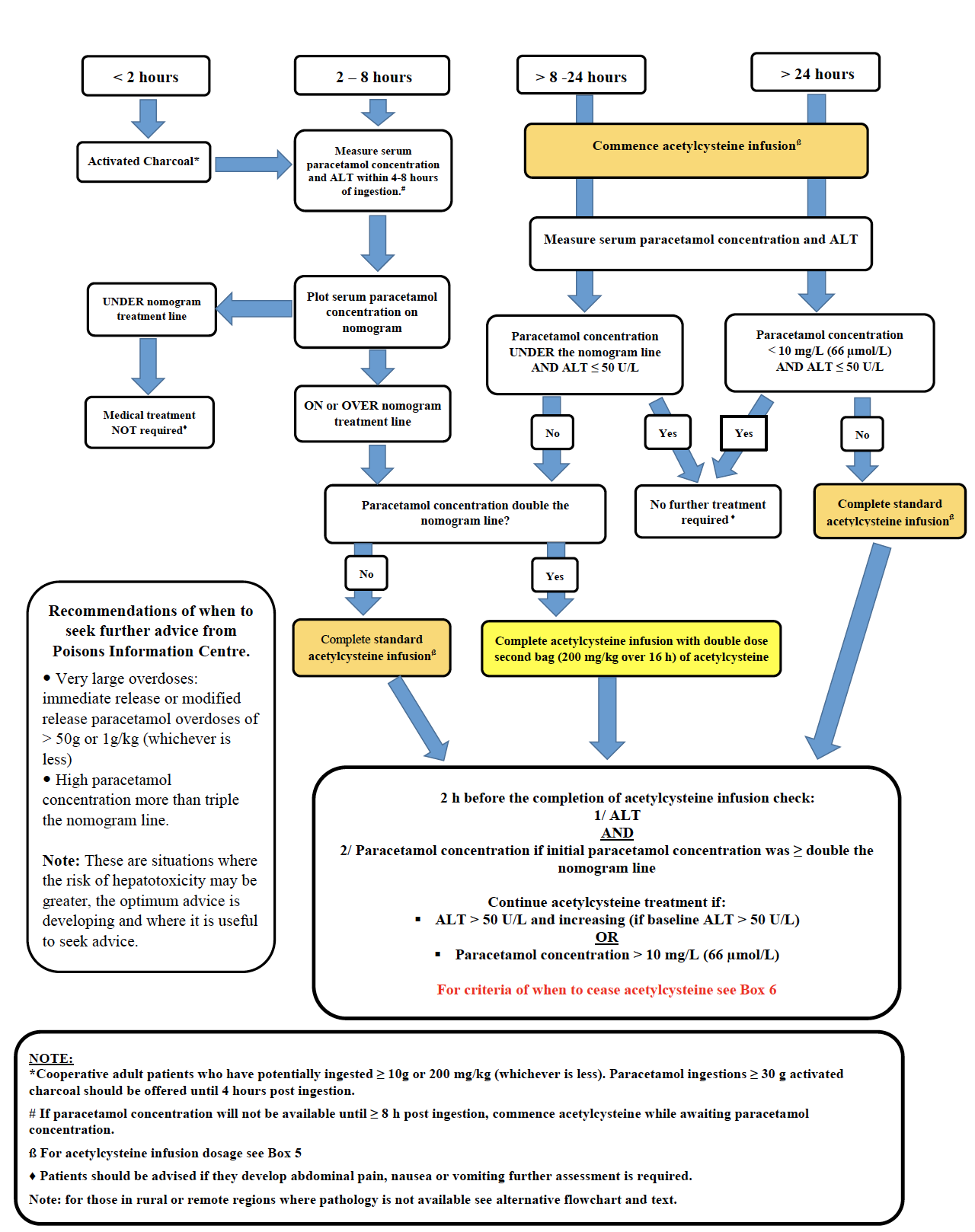
Adapted from Chiew A et al. Updated guidelines for management of paracetamol poisoning in Australia and New Zealand.
MJA. 2019.
Modified release paracetamol ingestions
Investigations
- Paracetamol concentration
- Initial concentration >4 hours post-ingestion
- Repeat concentration 4 hours later to guide acetylcysteine dose and need for further decontamination
- Baseline LFT with initial paracetamol concentration
- INR if more than 24 hours post ingestion or abnormal ALT
Acute management
Decontamination
- Activated charcoal has a very limited role in treatment and should only be used in consultation with a toxicologist
- Should be offered in modified release paracetamol ingestion
- >200 mg/kg or >10 g up to 4 hours post-ingestion
- >500 mg/kg or >30 g up to 24hrs post-ingestion
- Serial paracetamol concentrations are rising
Ongoing care and management
- Ingestions involving longer-acting forms of paracetamol (modified release, eg Panadol Osteo) should be discussed with Poisons Information Centre or a clinical toxicologist
- See modified release (MR) paracetamol flowchart below, to be used in combination with nomogram and acetylcysteine infusion guide
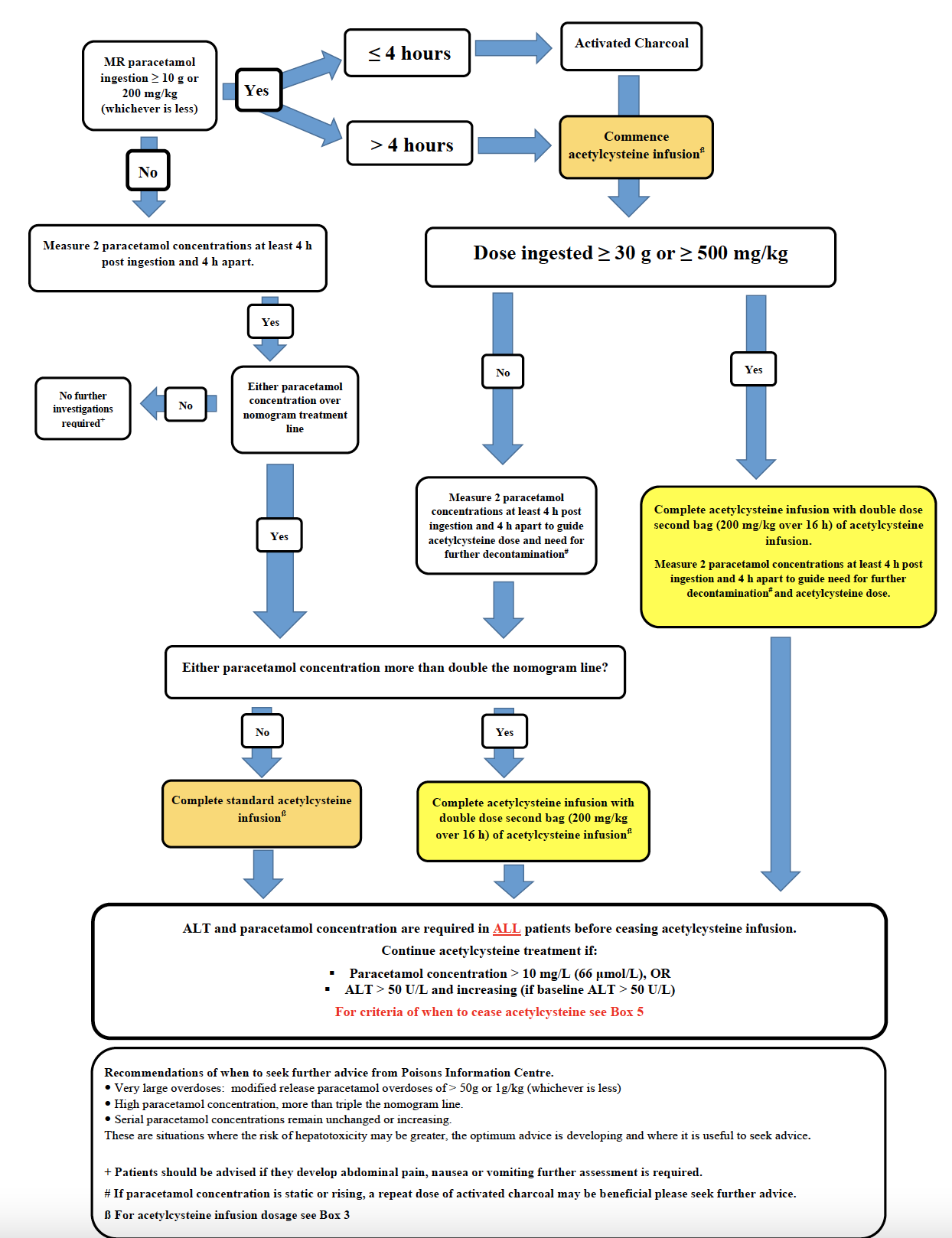
Adapted from Chiew A et al. Updated guidelines for management of paracetamol poisoning in Australia and New Zealand.
MJA. 2019.
Repeated supra-therapeutic paracetamol ingestion
Do not use paracetamol nomogram to interpret paracetamol concentrations for repeated supra-therapeutic ingestions
Investigations
- Serum paracetamol concentration and ALT should be sent if ingestion is:
- >200 mg/kg or >10 g (whichever is less) over a single 24-hour period
- >300 mg/kg or >12 g (whichever is less) over a single 48-hour period
- Greater than a daily therapeutic dose per day for more than 48 hours in patients who also have abdominal pain, nausea or vomiting
Acute management
- Commence acetylcysteine if
OR
- Serum paracetamol concentration >20 mg/L (132 micromol/L)
- Discuss with Poisons Information Centre or a clinical toxicologist
- See repeated supra-therapeutic ingestion management flowchart below
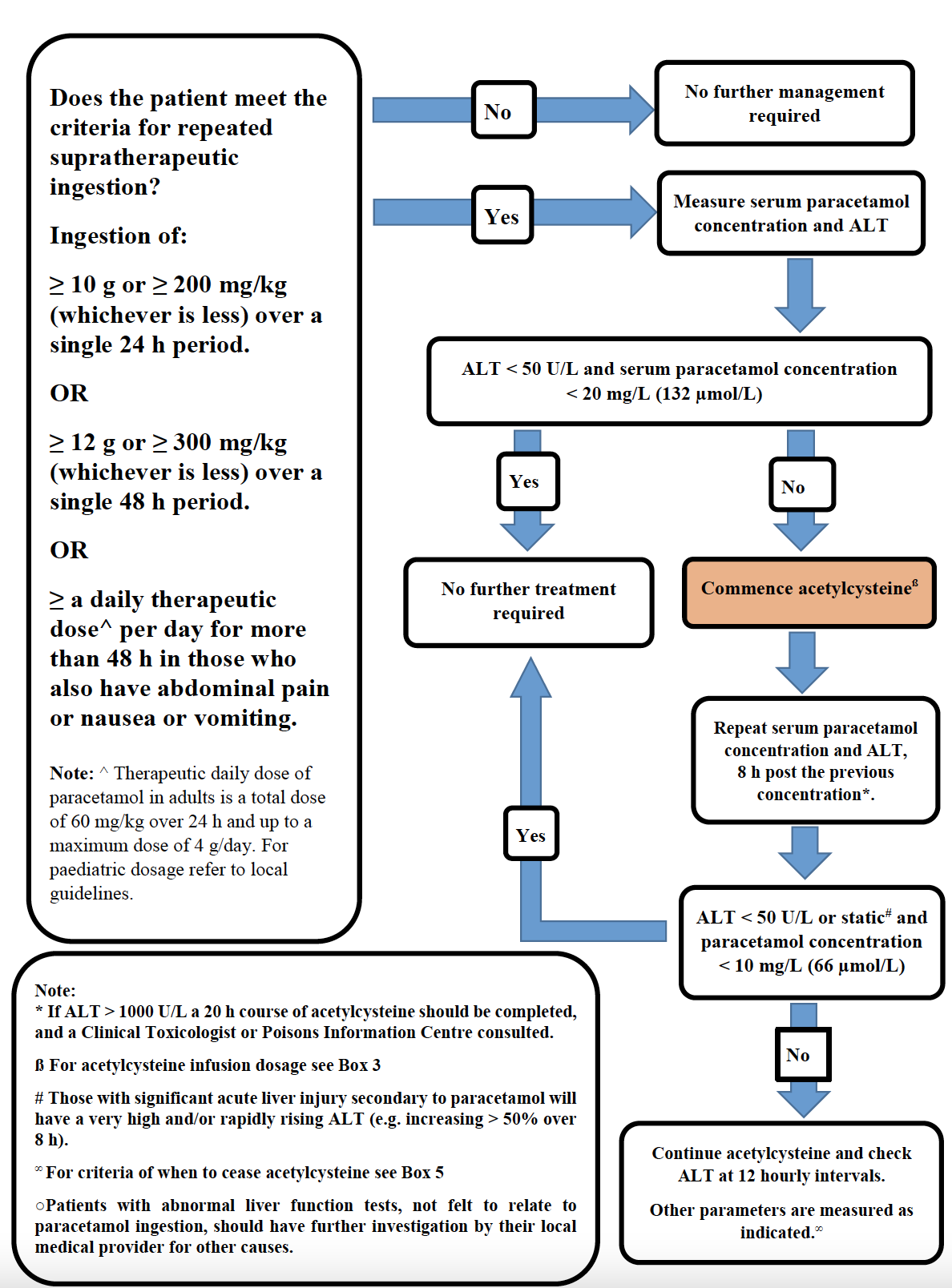
Adapted from Chiew A et al. Updated guidelines for management of paracetamol poisoning in Australia and New Zealand. MJA. 2019.
Acetylcysteine infusion instructions
Dose
Acetylcysteine is administered as a 2-stage infusion giving a total dose of 300 mg/kg:
- 200 mg/kg over 4 hours in crystalloid solution 7 mL/kg (up to 500 mL) intravenously
- 100 mg/kg over the next 16 hours in crystalloid solution 14 mL/kg (up to 1000 mL) intravenously
- For massive paracetamol exposures: double the dose of acetylcysteine in the second acetylcysteine infusion if the paracetamol concentration is >2 times the nomogram
Dose calculated based on actual body weight. For patients >110 kg, calculate the dose based on 110 kg body weight
Administration
- Acetylcysteine is compatible for dilution with any fluid. Given the volume required, dilution with a maintenance fluid, such as 0.9% sodium chloride in 5% glucose, is recommended
- Include the volume of acetylcysteine in the TOTAL volume of the infusion to avoid under-dosing
- volumes specified in tables below are TOTAL volumes, ie acetylcysteine volume plus fluid volume combined
- Additional maintenance fluids can be given if required, or acetylcysteine may be administered in larger volume bags if more convenient
Side effects
- Acetylcysteine infusion can be associated with:
- Gastrointestinal upset, eg nausea, vomiting, abdominal pain, diarrhoea
- Nonimmunologic anaphylaxis (formerly known as anaphylactoid reaction)
- Symptoms may include flushing, rash, itch, wheeze, shortness of breath, angioedema, hypotension
- In these cases, cease the infusion, give promethazine. Recommence infusion after 30 minutes at half the previous rate. Slowly increase to the full rate over 30 minutes.
- Previous non-IgE mediated anaphylactoid reaction does not preclude use in future presentation
Monitoring
18 hours after the initiation of the acetylcysteine infusion (2 hours before completion of the second bag), send bloods for:
- Paracetamol concentration
- ALT
If delayed presentation more than 24 hours post ingestion, also measure:
- INR
- Venous blood gas
- Urea, electrolytes, creatinine (5% of patients with paracetamol toxicity will develop acute renal injury)
Consult Poisons Information Centre or clinical toxicologist if continued treatment is required beyond the standard 20 hour course
Discontinuation
DO NOT cease acetylcysteine early without speaking to the Toxicology team, except in cases of anaphylactoid reactions (see above). Any modifications should be discussed with Poisons Information Centre
Acetylcysteine infusion should only be discontinued after completion of a 20-hour course if:
- ALT is normal
- Paracetamol concentration <10 mg/L (66 micromol/L)
- Patient is clinically well
All other cases should be discussed with Poisons Information Centre or a clinical toxicologist
Acetylcysteine guidelines by weight
For children or adolescents >40 kg (recommended ceiling weight 110 kg):
| Infusion stage |
Acetylcysteine dose |
Dilute to |
Rate and duration |
First |
200 mg/kg |
TOTAL volume 500 mL |
125 mL/hr for 4 hours
Infuse entire bag |
Second |
100 mg/kg |
TOTAL volume 1000 mL |
62.5 mL/hr for 16 hours
Infuse entire bag |
For children 20-40 kg body weight:
| Infusion stage |
Acetylcysteine dose |
Dilute to |
Rate and duration |
First |
200 mg/kg |
TOTAL volume 250 mL |
62.5 mL/hr for 4 hours
Infuse entire bag |
Second |
100 mg/kg |
TOTAL volume 500 mL |
31.25 mL/hr for 16 hours
Infuse entire bag |
NOTE: this results in a total of 750 mL of fluid which is inappropriate for smaller children.
For children ≤20 kg body weight:
| Infusion stage |
Acetylcysteine dose |
Dilute to |
Rate and duration |
First |
200 mg/kg |
TOTAL volume 250 mL** |
62.5 mL/hr** for 4 hours
Infuse entire bag |
Second |
100 mg/kg |
TOTAL volume 250 mL** |
15 mL/hr** for 16 hours
Infuse entire bag |
**For infants, even smaller volumes may be required. Doses can be diluted in 100 mL bags if available (note: the entire dose must be administered over the specified time). For infants who require smaller total volumes, contact hospital pharmacist for advice.
Infant example:
| Infusion stage |
Acetylcysteine Dose |
Dilute to |
Rate and Duration |
First |
200 mg/kg |
TOTAL volume 100 mL |
25 mL/hr for 4 hours
Infuse entire bag |
Second |
100 mg/kg |
TOTAL volume 250 mL |
15.7 mL/hr for 16 hours
Infuse entire bag |
Consider consultation with local paediatric team when
Admission should be considered for all children and young people with an intentional overdose
Consult the Poisons Information Centre 13 11 26 for advice
Consider transfer when
Children requiring care beyond the comfort level of the hospital
For emergency advice and paediatric or neonatal ICU transfers, see Retrieval Services
Consider discharge when
- No further acetylcysteine requirement and investigations are within normal limits (see Monitoring section)
- For deliberate ingestions, a risk assessment should indicate that the patient is at low risk of further self-harm in the discharge setting
Assessing risk and connecting to community services
- Prior to discharge, adolescents who present with deliberate ingestions need a risk assessment regarding the likelihood of further ingestions or other attempts to self-harm
- Assessment of other drug and alcohol use should also be undertaken
- If, after risk assessment, it is deemed safe to discharge a child or adolescent from hospital, but ongoing mental health or drug and alcohol needs are identified, they should be linked with appropriate services
Parent information
Poisoning prevention for children
Additional resources
Poisons Information Centre
Mental Health, Drug and Alcohol Services:
New South Wales
Child and Adolescent Mental Health Services: services delivered across NSW Health with referrals made via the NSW Mental Health Line (1800 011 511) for 24-hour advice, assessment referral information
Youth Health and Wellbeing: includes links to Assessment Guideline for providers caring for young people aged 12 – 24 years across settings, as well as links to other resources
Your room: information on alcohol and other drug use, including fact sheets (multiple languages), assessment tools and links to support services
Queensland
Child and Youth Mental Health Services: specialise in helping infants, children and young people up to age 18 years with complex mental health needs
Dovetail: provides clinical advice and professional support to workers, services and communities who engage with young people affected by alcohol and other drug use
Queensland Youth AOD Services Guide: created by Dovetail, this guide provides an overview of youth alcohol and other drug treatment services across Queensland. For help outside of hours, call the 24-hour Alcohol and Drug Information Service (ADIS) on 1800 177 833
Clarence St, Mater Young Adult Health Service: Youth drug and alcohol service
Victoria
Child & Adolescent Mental Health Services (CAMHS): Victorian government mental health services are region-based
Youth Support and Advocacy Service (YSAS): Outreach teams across Melbourne and regional Victoria for young people experiencing significant problems with alcohol and/or drug use
Victoria’s Youth Drug and Alcohol Advice (YoDAA): provides information and support for youth AOD needs or anyone concerned about a young person
Infoxchange Service Seeker: Search for local community support services eg local doctor, dentist, counselling services, drug and alcohol services
Last updated July 2023