See also
Fever in returned traveller
Febrile child
Key points
- Most influenza in healthy children is self-limiting and uncomplicated
- Some chronic illnesses predispose children to higher risk of severe disease, but up to half of severe cases occur in previously well children
- In hospitalised children with severe disease, antiviral treatment should be commenced empirically if less than 48 hours since the onset
- In an unwell child, presence of influenza does not exclude co-existing serious bacterial infection
Background
- Incubation period is 1–4 days (average 2 days)
- Hand and respiratory hygiene (eg covering the mouth when coughing) reduce transmission
- Infectious period is from 24 hours prior to onset of symptoms for 4–7 days of illness. This can be prolonged in young children or those with immunodeficiency
Assessment
Clinical features of uncomplicated influenza
- Fever (one third of children have fever without other symptoms)
- Rigors or chills
- Respiratory symptoms: rhinorrhoea, sore throat, cough, croup, otitis media
- Headache
- Myalgia, arthralgia
- Lethargy, malaise
- Less common: conjunctivitis, abdominal pain, nausea and vomiting
Younger children may be:
- less likely to have respiratory features
- more likely to have febrile seizures
- more likely to have gastrointestinal features
- more likely to have severe disease – see
Febrile child
Complications of severe influenza
- Pneumonia
- Secondary bacterial infection especially S.
aureus and S. pneumoniae
- Respiratory failure
- Neurological complications
- Encephalopathy / encephalitis
- Aseptic meningitis
- Guillain-Barre Syndrome
- Cerebellar ataxia
- Myositis and rhabdomyolysis
- Myocarditis and pericarditis
- Reye syndrome (associated with aspirin use)
Risk factors for severe disease – consider treatment (see flowchart)
- Severe chronic disease, particularly:
- neurological
- respiratory
- cardiac
- haematological eg sickle cell disease
- Genetic condition eg trisomy 21
- Premature infant
- Immunosuppression
Management
Immunisation status does not affect the management of children with suspected influenza
Investigations
- Consider testing where confirmation is likely to influence treatment decisions - see
flowchart
- Detection of influenza virus by nucleic acid (PCR) testing from appropriate respiratory specimen (nasal or throat swab or nasopharyngeal aspirate)
Treatment
Supportive care eg hydration and/or respiratory support
Admitted children should be isolated (or cohorted) - discuss with hospital infection control
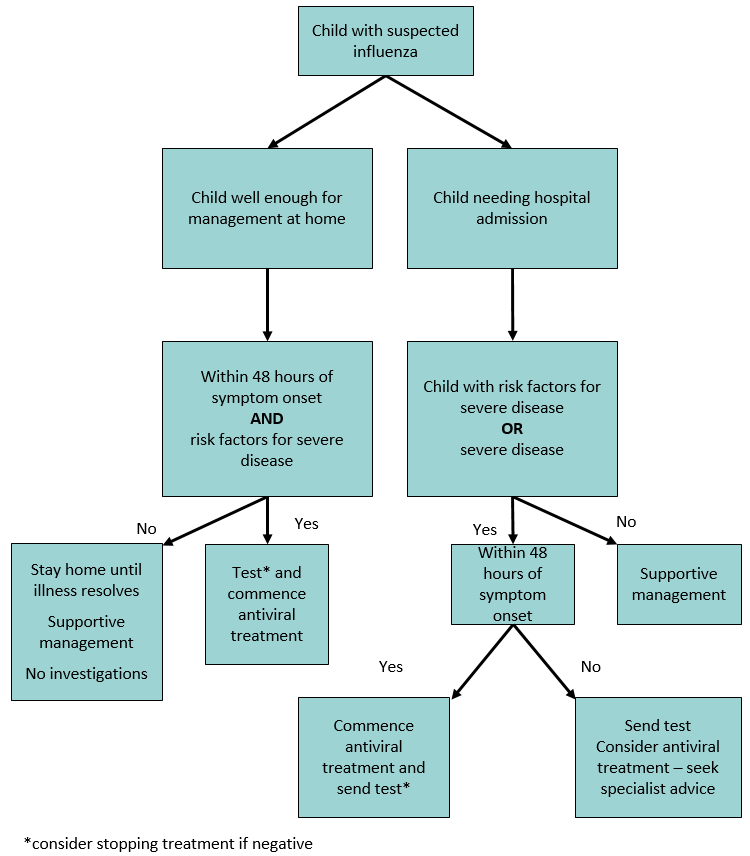
Clinical features at presentation - indications for treatment
Severe respiratory (eg pneumonia), neurological (eg encephalitis), cardiac (eg myocarditis, pericarditis), muscle (eg rhadomyolysis) or multi-organ failure
Antiviral
treatment
- Oseltamivir, zanamivir and peramivir are neuraminidase inhibitors that reduce influenza virus replication
- Oseltamivir is usually first line therapy (oral or nasogastric)
- Treatment may have a modest effect in terms of reducing duration of symptoms (1 day on average) and may prevent more severe disease
- Treatment is most effective if started within 48 hours of onset of illness
- Neuraminidase inhibitors have no role in treating other viral infections
Oseltamivir
- Oral capsule 30 mg, 45 mg, 75 mg or oral suspension 6 mg/mL
- Capsule contents may be dissolved in water (for use immediately)
- Side effects:
- headache
- nausea and vomiting (reduce by giving with food), twice as likely to have vomiting (from 4% to 8% of unwell children)
- For use in renal impairment – seek advice
Oseltamivir
dosage
| Weight |
Doses
(oral) |
Duration |
| Treatment |
| Birth (term)–12 months |
3 mg/kg/dose bd |
5 days |
| 1–18 years |
|
<15 kg |
30 mg bd |
| 15–23 kg |
45 mg bd |
| 23–40 kg |
60 mg bd |
| >40 kg |
75 mg bd |
| Prophylaxis |
| Birth (term)–12 months |
3 mg/kg/dose daily |
10 days |
| 1–18 years |
|
<15 kg |
30 mg daily |
| 15–23 kg |
45 mg daily |
| 23–40 kg |
60 mg daily |
| >40 kg |
75 mg daily |
*doses based on AMH
Peramivir
- Seek specialist advice – for inpatient use only
- Only children over 2 years
- IV preparation – used when enteral route not appropriate
Consider consultation with local paediatric
team when
Child has suspected influenza and risk factors
Consider transfer when
- Child requiring care above the level of comfort of the local hospital
- Child has severe disease
For emergency advice
and paediatric or neonatal ICU transfers, see Retrieval Services
Consider discharge when:
Child is well and education provided to monitor for complications
Parent information
RCH Kids Health Info Fact Sheet
NSW Children’s Hospital Fact Sheets
Additional notes
Influenza vaccine
- The best available preventative measure against influenza
- Recommended for all people aged >6 months annually
- Strongly recommended and funded for children ≥6 months and
<5 years
- Strongly recommended and funded for all children with Aboriginal or Torres Strait Islander background, children with risk factors for severe influenza and pregnant women
- More information is available from the
Australian Immunisation Handbook and the
ATAGI annual position statement.
Prophylaxis of contacts
In hospital contact:
- significant exposure is classified as being within 1 metre >15 mins without personal protective equipment
After significant exposure, suggest oseltamivir (prophylaxis) for children and family members if:
- they have risk factors
- household contacts have risk factors
- household contacts are:
- aged ≥65 years
- women who are pregnant or within 2 weeks after delivery
Last updated September, 2019