Introduction
Aim
Definition of Terms
Assessment
Managment
Potential Complications
Discharge Planning
Family Centered Care
Special Considerations
Companion Documents
Links
Evidence Table
References
Introduction
This guideline applies to neonates within the first two weeks of life.
Phototherapy is the use of visible light to treat severe jaundice in the neonatal period. Approximately 60% of term babies and 85% preterm babies will develop clinically apparent jaundice, which classically becomes visible on day 3, peaks days 5-7 and resolves by 14 days of age in a term infant and by 21 days in the preterm infant. Treatment with phototherapy is implemented in order to prevent the neurotoxic effects of high serum unconjugated bilirubin. Phototherapy is a safe, effective method for decreasing or preventing the rise of serum unconjugated bilirubin levels and reduces the need for exchange transfusion in neonates.
Aim
This guideline provides health care providers with information to understand the causes of neonatal jaundice, the rationale for the use of phototherapy and outlines the care of neonates receiving phototherapy in order to enhance effective phototherapy delivery and minimise complications of phototherapy.
Definition of Terms
- Jaundice: the yellow appearance of the skin that occurs with the deposition of bilirubin in the dermal and subcutaneous tissues and the sclera.
- Bilirubin: the orange-yellow pigment of bile, formed principally by the breakdown of haemoglobin in red blood cells at the end of their normal life-span. Neonate’s bilirubin production rate is double that of adults and their clearance of bilirubin is reduced, hence the importance of monitoring levels and detecting jaundice in this early post-natal period.
- Bilirubinaemia: the presence of bilirubin in the blood.
- Hyperbilirubinaemia: the excess of bilirubin in the blood.
- Unconjugated Hyperbilirubinaemia: most common form of neonatal hyperbilirubinemia. The bilirubin has not been metabolised and hence cannot be excreted via the normal pathways in the urine and bowel. Unconjugated bilirubin binds with lipids and albumin, and results in the yellow appearance of the skin and sclera. Unconjugated bilirubin can cross the blood-brain barrier and cause neurotoxic effects.
- Conjugated Hyperbilirubinaemia: less common in neonates. The bilirubin has been metabolised and is water soluble, but accumulates in the blood usually due to hepatic dysfunction. Conjugated bilirubin does not cross the blood-brain barrier.
- Serum Bilirubin (SBR): reports the unconjugated and conjugated bilirubin levels. This is the usual specimen requested by Medical staff on the pathology slip at RCH.
- Breast milk jaundice: develops within 2-4 days of birth, is most likely related to limited fluid intake as breast milk supply is established, may peak at 7-15 days of age and may persist for weeks.
- Phototherapy: a treatment for jaundice where the exposure of skin to a light source converts unconjugated bilirubin molecules into water soluble isomers that can be excreted by the usual pathways. Blue-green light is most effective for phototherapy as it both penetrates the skin and is absorbed by bilirubin to have the photochemical effect.
- Bilirubin encephalopathy: the acute manifestations of bilirubin toxicity seen in the first few weeks after birth. Signs include lethargy, hypotonia and poor suck progressing to hypertonia, opisthotonos, high-pitched cry and eventually to seizures and coma.
- Kernicterus: the pathogenic diagnosis characterised by bilirubin staining of the brain stem and cerebellum. Also the term used to refer to chronic bilirubin encephalopathy. Clinical findings include cerebral palsy, developmental and intellectual delay, hearing deficit, dental dysplasia and oculomotor disturbances.
- Single Light: One Natus neoBLUE® TM LED phototherapy unit (mini or standard) OR BiliSoft™ Biliblanket
- Double Lights: Two neoBLUE LED phototherapy unit’s (mini or standard) or One neoBLUE® LED phototherapy unit (mini or standard) + One BiliSoft™ Biliblanket.
- Triple Lights: Three neoBLUE® LED phototherapy unit’s (mini or standard) or Two neoBLUE® LED phototherapy unit (mini or standard) + One BiliSoft™ Biliblanket
**All phototherapy units are to be set on high intensity at all times, regardless of the amount of units in use. This ensures delivery of adequate amounts of blue light via light emitting diodes (LEDs). Therefore, a single unit is classified as a single light and single, double or triple lights refers to the amount of units not the intensity setting.
**As per Natus neoBLUE® LED phototherapy in-service guide (available on the intranet), mini neoBlue® LED phototherapy units deliver the same intensity as the standard unit set on high intensity; the only difference is in the surface area coverage.
Assessment
- Please select the correct Bilirubin chart (EMR Bilirubin Phototherapy Activity tipsheet), taking in to account gestational age, weight and risk factors which include sepsis, haemolysis, acidosis, asphyxia, hypoalbuminaemia. This is because the risk of developing kernicterus increases in the presence of the above risk factors.
- The Bilirubin charts onto which total SBR is plotted are for the first 7 days of life. After the first 7 days continue utilising these charts, as levels plateau and can continue to be documented.
Assess general skin colour and skin temperature whenever measuring and recording vital signs. Ensure the Phototherapy tick box in the EMR Flowsheets is activated and document time of commencement and cessation.
- Document hourly the type and number of light banks and the presence of eye protection.
- Obtain blood sample to measure serum bilirubin levels (either venous, arterial or capillary (RCH Specimen Collection). Ensure the lights are turned off during sampling so accuracy of current blood levels can be attained. Initially SBR levels may need to be assessed every 4-6 hours until reduction. Follow medical advice and ordering of SBR levels and plot on appropriate line of the chart.
- Observe for signs of lethargy and poor feeding
- Kramer’s Rule - Is a quick non-invasive method of assessing the degree of Jaundice. Blanch the skin in each of the five zones shown below, observe the colour of the blanched skin (will be yellow if jaundiced) - it gives you an indication of what the bilirubin level may be. The zones show the natural progression of increasing jaundice levels. It should only be used as a guide, serum bilirubin levels should always be obtained.
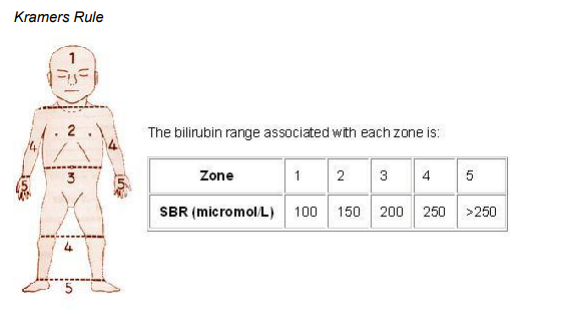
Kramer L.I., (1969), Advancement of Dermal Icterus in the Jaundiced
Newborn, Amer J Dis Child, 118: 454-458.
Investigations
- Initial SBR measurement should be requested based on clinical observation and the following factors:
- any neonate <24 hours age with clinically apparent jaundice
- any neonate where there is clinical doubt about the degree of jaundice
- any unwell neonate with jaundice
- any neonate with risk factors for jaundice (ABO/ Rh incompatibility, sepsis, acidosis, asphyxia, hypoalbuminaemia)
- Ongoing SBR measures should be repeated at intervals depending on the initial level and rate of rise. For example: 6 hourly measures may be required if the level is very high and the neonate is being treated with multiple phototherapy lights, follow Medical advice.
- A SBR should be collected 24 hours post cessation of phototherapy lights to check for rebound hyperbilirubinaemia.
- For neonates at increased risk of clinically significant rebound hyperbilirubinaemia including those born less than 37 weeks gestation, those not feeding optimally or those with haemolytic disease, a request for a SBR collection may occur within 12-24 hours of ceasing phototherapy.
- Further Bloods and investigations include
- Maternal and infant blood type
- Direct Coombs test
- Haemoglobin
- Full blood count for red cell morphology; reticulcyte, haematocrit and platelet counts and white blood cell differential
- Urinalysis for reducing substances
- Sepsis screen if sepsis suspected
- G6PD and galactosaemia screens if suspected
- Serum thyroxine and thyroid-stimulating hormone levels
Risk Factors
- Mothers with a positive antibody screen
- A family history of G6PD deficiency
- A previously affected sibling
- Cephalhaematoma, bruising and trauma from instrumental birth
- Delayed passage of meconium
- Prematurity
- Dehydration
- Inadequate breastfeeding
- ABO/Rh incompatibility
Nutrition
Breastfed babies who require phototherapy should continue to breastfeed unless clinically contra-indicated due to other pathology; the neonate’s sucking, attachment and mother’s milk supply should be monitored. All feeds should be given via a bottle or NGT if feeding is deemed safe. Neonates who are receiving enteral feeds of EBM or infant formula should continue to do so. The total fluid intake (TFI) for a 24 hour period may need to be increased by at least 10% to account for insensible fluid loss when a neonate is receiving phototherapy however this should be guided by hydration status and electrolyte monitoring.
Parenteral nutrition and IV fluids should continue as ordered and may also need to be increased by 10% to account for insensible fluid loss. In the case of infants nearing exchange transfusion level, the infant should not come out of phototherapy to feed as this is a medical emergency.
Phototherapy Management
Nutrition
Breastfed babies who require phototherapy should continue to breastfeed unless clinically contra-indicated due to other pathology; the neonate’s sucking, attachment and mother’s milk supply should be monitored. In the case of infants nearing exchange transfusion level, the infant should not come out of phototherapy to feed as this is a medical emergency. All feeds should be given via a bottle or NGT if feeding is deemed safe
Neonates who are receiving enteral feeds of EBM or infant formula should continue to do so. The total fluid intake (TFI) for a 24 hour period may need to be increased by at least 10% to account for insensible fluid loss when a neonate is receiving phototherapy however this should be guided by hydration status and electrolyte monitoring.
Parenteral nutrition and IV fluids should continue as ordered and may also need to be increased by 10% to account for insensible fluid loss.
Phototherapy
- Commence phototherapy once SBR is greater than the appropriate
reference range for neonate’s gestation/weight and presence of risk
factors.
- Neonates
should be nursed naked apart from a nappy under phototherapy and will need
to be nursed in an overhead radiant warmer bed (ATOM or LEO) or Isolette
to maintain an appropriate neutral thermal environment (Ward management of a neonate, Assisted thermoregulation). In severe cases, the
nappy may need to be removed and a urine bag applied to maximise skin
exposure.
- Positon
phototherapy units no more than
30.5cm from the patient. neoBLUE® LED phototherapy unit can be
positioned as close as 15cm to
patient. Refer to specific phototherapy units manufacturing guidelines
for more details
- When
patient is being nursed on an ATOM bed, the use of the neoBLUE® Mini
phototherapy units are used, where practical, together with the BiliSoft™
BiliBlanket, to ensure not overheating the patient.
- BiliSoft™
BiliBlanket’s require the use of Phototherapy Disposable Pad Covers and
are available form Butterfly Ward. The correct way to position the BiliSoft™
Biliblanket is with the fibres facing downwards and the paper on top (see
image below).
- Expose as much of the skin surface as possible to the phototherapy
light. To maximise skin exposure, dress the baby in a nappy and their
protective eye covers only.
- Cover the eyes with appropriate opaque eye covers e.g. Natus
Biliband® Eye Protector (available from Butterfly ward).
- Ensure eye covers are removed 4-6 hourly for eye care during infant
cares or feeding. Observe for discharge/infection/damage and document any
changes.
- Daily fluid requirements should be reviewed and individualised for
gestational and postnatal age.
- Maintain a strict fluid balance chart.
- Breast feeds may need to be limited to 20 minutes if bilirubin
level is high to minimise amount of time out of the lights
- Monitor vital signs and temperature at least 4 hourly. Hourly if patient is nursed in isolette, ATOM or LEO
radiant warmer
- Cover
lipid lines with light resistant, reflective tape to
avoid peroxidation
- Ensure
that phototherapy unit is turned off during collection of blood for SBR
levels, as both conjugated and unconjugated bilirubin are photo-oxidized
when exposed to white or ultraviolet light.
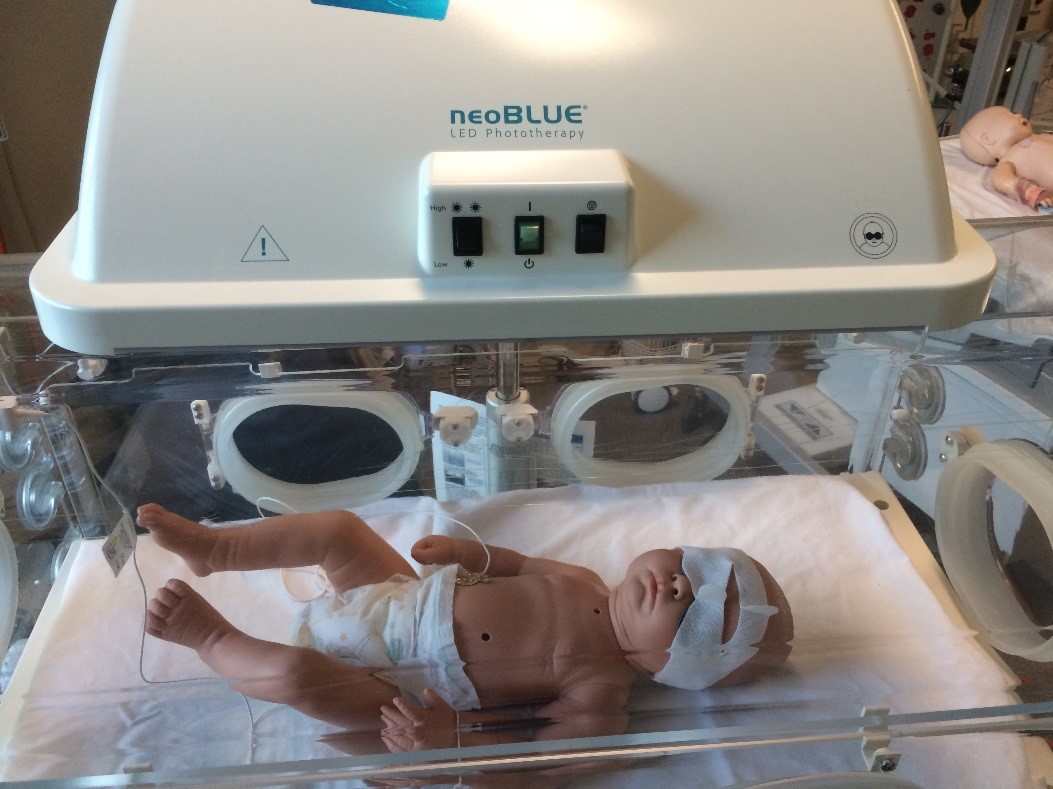
Picture taken at RCH

Picture taken at RCH
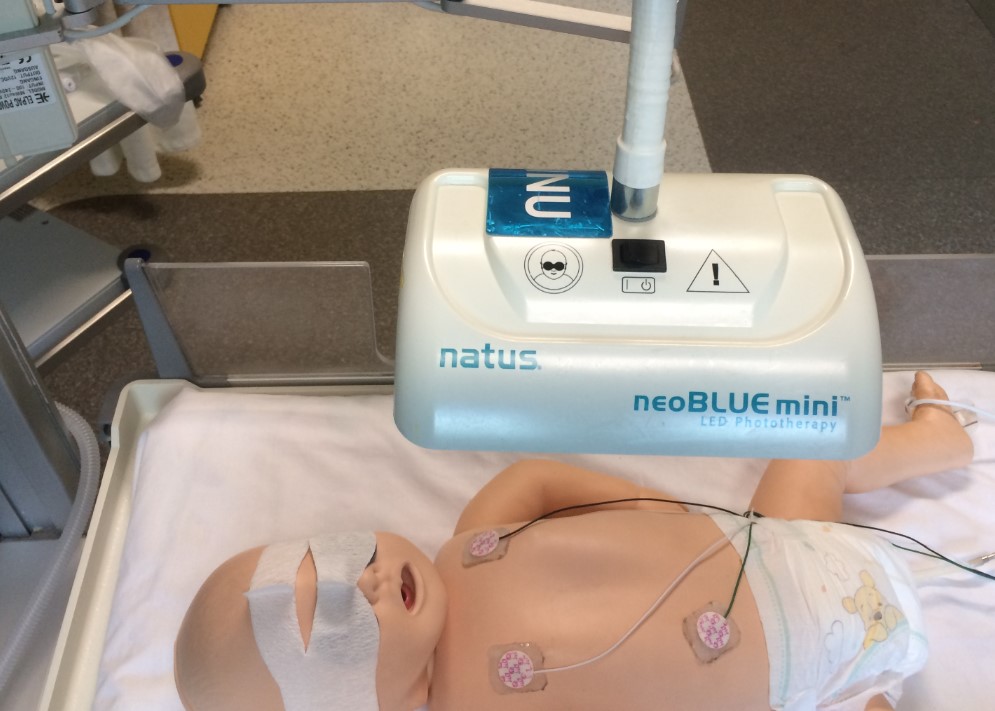
Picture taken at RCH
Potential Complications
- Overheating – monitor neonate’s temperature
- Water loss from increased peripheral blood flow and diarrhoea (if present)
- Diarrhoea from intestinal hypermotility
- Ileus (preterm infants)
- Rash
- Retinal damage
- ‘Bronzing’ of neonates with conjugated hyperbilirubinaemia
- Temporary lactose intolerance
Discharge planning and community-based management
Documentation in the neonates discharge letter and
Child Health Booklet should include details about SBR levels and duration of
phototherapy treatment. Normal hand hygiene should be attended to during care
of neonate receiving phototherapy. More details on the neoBLUE® LED lights can be found in the definition of terms.
Family Centered Care
Explain to parents the need for and actions of phototherapy, particularly in relation to the need for skin surface to be exposed to the phototherapy light, and hence the need to care for neonates receiving phototherapy to be nursed in a neutral thermal environment. Potential complications of phototherapy and the need for protective eye coverings during phototherapy treatment should be explained. The need for measuring SBR and other blood should also be explained.
Neonates receiving phototherapy (where there are no other contraindications) can have brief periods where the phototherapy is ceased so that they can be cuddled/breastfed and have their eye covers removed for parent-baby interaction to occur.
Special Considerations
- Infection control
- Patient safety alerts
- Potential adverse events
Companion Documents
Links
Evidence Table
Click here to view the evidence table for this guideline.
Please remember to read the
disclaimer.
The development of this nursing guideline was coordinated by Marley Stewart, RN, Butterfly, and approved by the Nursing Clinical Effectiveness Committee. Updated November 2022.