Pain in palliative care
Until recently, it was widely believed that children, particularly young children, experienced less pain than adults. Many clinical studies have negated these myths showing that children and adults experience similar degrees of pain. ᆴ1
One of the great fears of children and families in the setting of terminal illness is that of unrelieved pain. However in the vast majority of circumstances it is possible to effectively control pain with analgesic/pain relieving medications. Opioid addiction is
another concern for families, and some health professionals. Again, this fear can be allayed because it has been shown that patients receiving properly titrated doses of analgesia do not become addicted. ᆴ2 Parents and health professionals sometimes confuse
tolerance (the need for escalating doses to achieve the same therapeutic effect) with addiction. A patient's need for increasing doses of opioids is related to tolerance and disease progression, and should not be seen as impending addiction. The dose should be
increased according to an individual's need. It is important to anticipate and discuss concerns and fears about the use of opioid analgesics early in the terminal phase of the child's illness to ensure that misconceptions are addressed.

Assessment of pain
Self-report of pain levels is the "gold standard" of pain assessment in older children and adults. The evaluation of pain in children however is dependent on the child's age and developmental stage. Pain measurement and pain assessment are not the same thing
and while it may be possible to determine the site of pain and make some estimation of its severity, understanding its character and radiation is often more difficult. Children need to understand what is being asked of them so wherever possible their own terminology
should be used, e.g. using the word 'sore' or 'hurt' instead of pain. The response they give might also depend on who is asking the question and what the consequences may be. Children who are fearful of receiving a painful injection or unpleasant tasting medication
may not admit to pain. Health professionals need to utilise a number of parameters to adequately assess pain in children. Simple observation is extremely useful as changes in activity level and behaviours such as irritability or withdrawal may indicate
discomfort. It is important to remember however that the behaviour of a child in chronic pain differs from that of a child in acute pain. The latter tend to be more demonstrative and vocal while children in chronic pain may appear quiet, withdrawn, lack interest
in activities or surroundings, be reluctant to move, have clingy behaviour or whinging, or have difficulty sleeping. ᆴ3
Pain assessment in children should be multidimensional taking into account:
Self report. For children over the age of four years a number
of useful pain scales exist. Examples include;
-
- Visual analogue scales eg. Pain ladders and thermometers
- Faces Pain Scale a series of faces depicting varying levels of discomfort ᆴ4
- Oucher scale a series of photographs depicting varying levels of ᆴ5discomfort
- Poker Chip Tool the child is asked to hand the examiner the number of poker chips which corresponds to the degree of pain (max 4)
- Body outline the child is asked to use colour to indicate the site and severity of their pain
- Pain questionnaires these are useful in older children and adolescents
- Parental report. Parents know their children very well and are able to provide valuable interpretations of behaviour. However, parents tend to under-estimate pain in their children so their observations should never form the sole basis of pain assessment.
ᆴ6
- Medical staff observations of the child's behaviour a range of age-appropriate observation scales have been devised and validated scientifically for acute pain assessment but are also used for children with chronic pain.
- Changes in blood pressure and heart rate may provide some indication of pain but may be influenced by a number of other factors especially anxiety/fear.
- Other influences such as past pain experiences and coping strategies, the meaning of the pain to the child and family and their emotional state.

Principles of pain
management
- Comprehensively assess the pain and address all the
factors which may be contributing to the pain experience (eg. anxiety, other symptoms, family stress)
- Discuss the management strategy with the family andaddress potential anxieties
- Utilise a multimodal approach incorporating physical therapies such as massage, TENS machines and splints and non-pharmacological techniques such as music/play therapy, story book reading, hypnotherapy, guided imagery or direct to other therapies where
appropriate.
- Utilise the least invasive route of medication administration. The oral route is preferred as it causes minimal discomfort or disruption to the child and absorption from the gastrointestinal tract is good.
- In the palliative care of children, there is NO role for intramuscular analgesia.
- Administer analgesics regularly (by the clock) to control constant or frequent bouts of pain
- Minimise the number of doses required per day. Sustained release (or slow continuous) preparations of opioids are available in a variety of preparations.
- Plan ahead for exacerbations and crises and give immediate release medications for this or prior to pain inducing activities.
- Titrate the dose of opioid to effect. There is no maximum dose as such. The correct dose is that which relieves the patient's symptoms with minimal side effects.
- Prophylactically prescribe regular laxatives when using
opioids.
- Monitor the response to treatment, and review the opioid dose regularly.
- Seek advice if the pain is not quickly controlled
The World Health Organisation Analgesic Ladder has provided an excellent framework for the escalation of analgesia for many years. A more flexible approach is now advocated with management strategies customised according to the child's pain, the mechanism
involved, the child's particular needs and response to previous treatments. The entry level onto the ladder will be influenced by these factors. Treatment should be frequently reassessed and modified. An adjuvant analgesic agent (see 'Resistant' pain and
Adjuvant Analgesia.) can be added at any point on the ladder if indicated by the clinical circumstances eg. bone pain, neuropathic (nerve) pain, inflammatory pain.
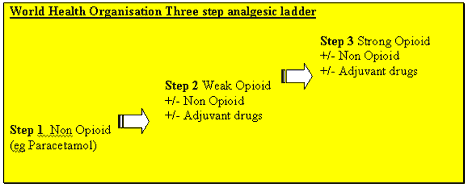
WHO Pain Ladder

Analgesic agents
Non-Opioids
Paracetamolᆴ is generally well tolerated in children and is very useful for mild pain (15mg/kg/dose
4/24 orally. Max 90 mg/kg/day or 6 doses) ᆴ7 It is available in a variety of oral preparations and for rectal use (not appropriate for neutropenic patients).
Non
steroidal anti-inflammatory drugs NSAIDs
These are powerful anti-inflammatory agents with analgesic and anti-pyretic activity. They may be helpful in alleviating musculoskeletal pain such as that induced by bone metastases and soft tissue inflammation and can be used in combination with opioids. There are a variety of NSAIDS on the market including
aspirin, naproxen, ibuprofen and diclofenac. Individuals may respond better to one agent than another. Side effects in children include nausea and vomiting, bronchospasm, gastric irritation and ulceration, and interference with platelet function. They should
not be used in children with
- thrombocytopenia or coagulation disorders
- gastrointestinal ulceration or bleeding
- significant renal impairment
Caution should be exercised if using NSAIDS in children with asthma.
Selective Cox 2
inhibitors or Coxibs
COX 2 inhibitors such as Celecoxib and Rofecoxib are equally analgesic when compared with non-selective NSAIDS. They offer certain advantages in that the risk of gastrointestinal bleeding is reduced, and they have no effect on platelet function.

Opioids
If paracetamol is ineffective in controlling pain, a weak opioid such as codeine phosphate oral 0.5 mg/kg/dose 4/24ᆴ7 may be used. Codeine however can result in severe constipation, and has a ceiling effect (that is, a maximum effect is achieved
beyond which further dose increases yield no benefit), so morphine is preferred in patients who are likely to require escalating doses. Morphine is still the "gold standard" against which all other opioid analgesics are measured, and is the drug of preference
unless there are circumstances that prohibit its use.
Where ongoing pain is expected, immediate release morphine in the appropriate dosage should be prescribed every 4 hours. Prn dosing of analgesia in the palliative care of children is not appropriate as "breakthrough pain" resulting from falling plasma
levels of morphine is very distressing to the child and family. Pain management is more effective if symptoms are anticipated and prevented with medication administration.
The starting dose of oral morphine for opioid nave children aged over six months is 0.2-0.5 mg/kg/dose
q4-6h orally. ᆴ8 Neonates have a higher relative volume of distribution and lower clearance than older children, leading to a longer half life. This, combined with the increased permeability of the blood brain barrier, means that infants may have a heightened
sensitivity to the respiratory depressant effects of opioids. A paediatric pain specialist or neonatologist should be approached for advice when dealing with infants under the age of 6 months. The onset of analgesic activity is approximately 30 minutes with peak
activity reached at 60-90 minutes. The average plasma half-life is 3 hours so steady state plasma concentration is achieved in 15 hours. Thus, the dose may be increased by 30-50% every 12-24 hours if pain control is suboptimal and more rapidly for a severe
exacerbation of pain. The child should be frequently reassessed to ensure pain is controlled with minimal side effects. Once the pain is under control and the appropriate 24-hour dose of morphine determined, a switch to a sustained release formulation of morphine
is appropriate. This continuous release form has a slower onset of action but a much longer duration and offers the advantages of less frequent dosing and improved sleep. Preparations are available for once and twice daily dosing. Provision for breakthrough pain should
be made with a 'rescue' dose of immediate release form of morphine. Breakthrough pain is a flare of pain that is above the usual or background pain that the child experiences.
Dosing regimens for continuous release morphine are calculated using the total daily amount of immediate release morphine required. For example, if 5 mg of oral morphine has been administered every 4 hours the total dose is 30 mg (6 x 5 mg) and this is converted to 15mg of sustained release morphine every 12
hrs. The "breakthrough" dose is equivalent to one sixth of the total daily dose of immediate release morphine, in this example 5mg.
If the child regularly requires repeated breakthrough or rescue doses of morphine (ie more than two per day), the dose of sustained release morphine needs to be increased. This is accomplished by calculating the total dose of morphine (both immediate and
sustained release) required in the previous 24 hours and dividing the dose. For example, if the child in the example above required 4 breakthrough doses of 5mg each then it would be appropriate to increase the dose of sustained release morphine to 25 mg BD. The
breakthrough dose of morphine will also need to be adjusted accordingly. To continue the example this would need to be increased to 8mg.

Algorithm for stabilisation of pain
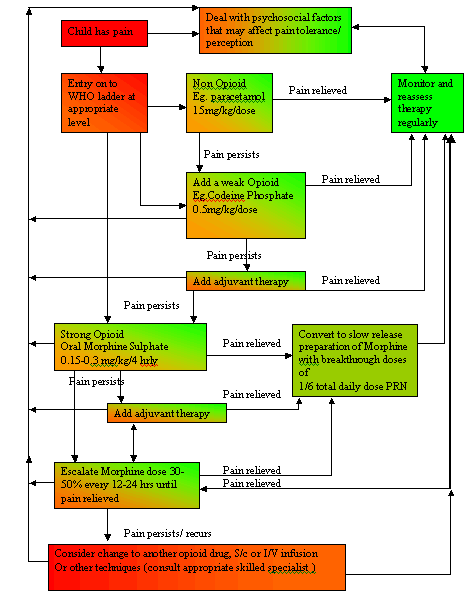

Alternative routes of delivery (subcutaneous, intravenous, spinal and epidural) may be required in the following circumstances
-
The child simply refuses oral medication
-
Vomiting, malabsorption
-
Inability to swallow, depressed conscious state
-
Severe or rapidly escalating pain may be more quickly controlled with intravenous or subcutaneous delivery of opioid after which oral medication may be commenced
-
Troublesome side effects due to accumulation of toxic metabolites. The parenteral or spinal route may offer a more favourable analgesia/toxicity profile.
The intravenous route is most practical in children with long-term venous access devices. For other children, the subcutaneous route is relatively simple and safe. In the setting of acute pain in an opioid nave patient aged over six months, theparenteral dose of morphine is 0.05-0.1mg/kg 4/24ᆴ8 For children who have been taking morphine orally, the
parenteral dose is one third of the oral dose. Subcutaneous infusions of morphine are manageable at home with appropriate equipment and support (see "parenteral infusions"). The 24-hour dose is determined by totaling the doses required in the previous
24 hours. For children switching to subcutaneous morphine from oral morphine, the dose is one third of the total oral dose. For example, a child who received a total oral dose of 72 mg, would require 24 mg per day of morphine as a continuous subcutaneous
infusion. This serves as a starting point only. The dose may need titrating up or down according to the patient's need. Breakthrough doses may be required and these are 1/6 of the total daily subcutaneous dose. In this example the breakthrough dose would be
4mg.
It is important when delivering morphine via the subcutaneous route that the volume of the infusion is considered, and every attempt made to keep infusion volumes as low as possible. There may be technical limitations with the type of syringe driver used that
make this difficult.
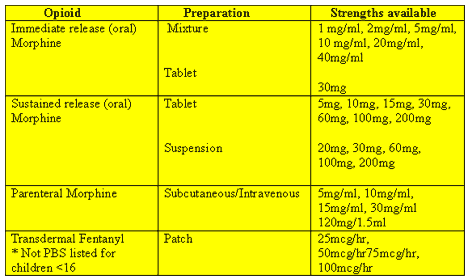
Fentanyl has become available in a transdermal formulation and provides an effective and convenient alternative to oral opioids in situations where pain is stable. Unfortunately, fentanyl patches are very expensive and not available on the PBS
for children under the age of 16. The lowest dose form of patch currently available is 2.5mg which delivers 25mcg/hr of transdermal fentanyl. As this equates to 1mg/hour of continuous IV morphine or 75mg of oral morphine, the child has to have a high opioid
requirement for this therapy to be an option. Transbuccal dosettes are also available for severe pain flares in patients on high dose opioid therapy and have the advantage of being rapidly acting. The lowest dosette size available is 200mcg and therefore is usually
only appropriate for prescription for adults. The assistance of paediatric pain specialists should be sought prior to commencing treatment with either of these forms of fentanyl.
A variety of other opioid medications are available and may offer advantages over morphine in certain situations. The advice of pain management specialists should be sought if pain is not controlled quickly.

Common opioid
preparations

Side effects of opioids
The most frequently encountered side effects are:
- Constipation is a very common and uncomfortable side effect of opiate use. A stool softener and aperients should be prescribed when opiates are commenced. Doses of stool softeners and aperients should be increased as the opioid dose increases.
- Nausea and vomiting can occur at the commencement of opioid therapy but can be relatively easily managed by prescribing an antiemetic. This side effect usually settles down after 2-3 days.
- Pruritus is not an uncommon side effect and is usually relieved with an antihistamine.
- Sedation often occurs at the commencement of therapy with opioids and whenever the dose is increased. Families may need to be warned of this effect as they may interpret it as a worsening of their child's condition. It is possible to reassure
families that once a stable dose has been achieved drowsiness decreases and children are usually active, alert and pain free. Sometimes stimulant therapy may be required during the day using oral agents such as caffeine or methylphenidate (which also has
synergistic analgesic properties with opioids).
- Respiratory depression can occur but is very unlikely if doses are carefully titrated. The effects upon breathing pattern in patients who are experiencing air hunger is beneficial.
- Nightmares a night time dose of haloperidol may be helpful
- Euphoria, dysphoria
- Urinary retention
- Physical dependence -opioids should be weaned and not ceased abruptly or a withdrawal reaction will occur.
- Tolerance see above. This is managed by increasing the dose.
Depending on the circumstances, side effects can be treated with appropriate medication or managed expectantly as many will settle as tolerance develops. If persistent, a reduction in dose, switch to an alternative opioid or change to a different route of delivery
may be required.
The management of respiratory depression associated with opioid use warrants special mention and may vary according to the child's overall condition. Drowsiness and respiratory slowing may be part of the dying process and in the terminal setting no specific
intervention may be required. In the early phases of palliative care where the child may enjoy a reasonable quality of life, active intervention may be warranted. Excessive slowing of respiration rate may be managed by rousing the child, slowing the rate of the
opioid infusion, administering oxygen as required and if necessary giving small doses of naloxone 0.01-0.1
microgram/kg every few minutes. Caution is required as rapid reversal may cause withdrawal, hypotension or the reemergence of severe pain.
Patients with significant hepatic or renal dysfunction should start at lower initial doses and be monitored closely for side effects as the drug and its metabolites may accumulate and result in respiratory depression. An alternative opioid such as fentanyl
may be more appropriate for patients with renal failure.

"Resistant" pain and Adjuvant therapy
Most pain can be controlled with adequate doses of opioid medication and much of what is regarded as "resistant" pain can be managed with a dose increase or an alternative route of delivery (eg. parenteral, spinal). In some circumstances however, the
addition of an adjuvant agent can greatly enhance pain control by targeting particular pain mechanisms. Adjuvants include antispasmodics, anxiolytics, corticosteroids, antidepressants, anti-epileptics, clonidine, ketamine, baclofen and others. For example, the addition of non-steroidal anti-inflammatory drugs may
help in relieving pain due to bony or soft tissue metastases. Neuropathic pain has characteristic features (eg electric shock-like quality, lancinating, burning, paresthesia) and may respond to antidepressants or antiepileptic agents.Amitriptyline 1-2mg/kg oral nocte ᆴ8 is a useful
agent and may be especially helpful for children with nocturnal pain, neuropathic pain or sleeping difficulties.
Nerve blocks or ablation, the use of chemotherapy or radiotherapy and the use of opioids delivered via the intrathecal or epidural routes are all techniques that may be of benefit in controlling pain in difficult circumstances. These techniques should be discussed with an appropriate specialist skilled in their
application prior to discussion with the family.

Other therapies for pain
The provision of accurate information to patients and families about their symptoms and treatment will greatly allay their fears and help with compliance and the control of pain. For children with chronic illness, minimising pain should be a priority from the time
of the first procedure. The use of topical anaesthetics and other strategies will help prevent bad pain memories and reduce their detrimental effect on experiences during future procedures.
Children may be greatly empowered and assisted by the use of complementary techniques such as acupuncture, guided imagery, relaxation training, meditation and distraction (stories, videos, music, bubble blowing, singing). Children can also be taught
techniques to use during painful procedures and staff members with expertise in this area are available to provide guidance in this area.
A range of physical therapies may be of use to the child in pain. Touch, massage warmth, cold and electrical therapies are all used in the management of various types of pain.

Acknowledgements
The authors gratefully acknowledge the kind assistance of Dr. Greta Palmer, anesthetist Royal Children's Hospital, Melbourne in reviewing this manuscript.
References
1/ Anand KJS, Grunau RVE, Oberlander TF. Developmental character and long-term consequences of pain in infants and children. Child Adolesc Psychiat North Am 1997; 6: 703.
2/ Woodruff R. Palliative Medicine: Symptomatic and supportive care for patients with advanced cancer and AIDS. Asperula. Melbourne.1993
3/ Gauvain-Piquard A, Rodary C, Lemerle J. L'atonie psychomotrice: signe majeur de doleur chez l'enfant de moins de 6 ans. J Parisiennes Pediatrie 1988;249-52.
4/ Bieri D, Reeve RA, Champion GD, et al. The faces pain scale for the self-assessment of the severity of pain experienced by children: development, initial validation, and preliminary investigations for ratio scale properties. Pain 1990; 41: 139-150.
5/ Beyer JE, Wells N. The assessment of pain in children. Pediatr Clin North Am 1989; 36: 837-54.
6/ Chambers CT, Reid G, Craig KD, McGrath PJ, Finley GA. Agreement between child and parent reports of pain. Clin J Pain 1998; 14: 336-342.
7/ Therapeutic Guidelines, Palliative Care. Therapeutic Guidelines.Melbourne.
2001.
8/ Kemp CA, McDowell JM (Eds). Paediatric Pharmacopoeia. 13th Edition. Royal Children's Hospital. Melbourne. 2002.
Resources
McGrath P. Pain in Children: Nature, Assessment and Treatment. Guildford Press. New York.1990.
World Health Organisation and International Association for the Study of Pain. Cancer Pain Relief and Palliative Care in Children. World Health Organisation. Geneva. 1998.
Franck LS, Greenberg CS, Stevens B. Pain Assessment in Infants and Children. Pediatr Clin North Am 2000; 47: 487-512.
Therapeutic Guidelines, Palliative Care. Therapeutic Guidelines. Melbourne 2001.
Royal College of Paediatrics and Child Health. Prevention and Control of Pain in Children: a Manual for Health Care Professionals. British Medical Journal Publishing Group London 1997.
Goldman A. ABC of Palliative care: Special problems of children. British Medical Journal 1998; 316: 49-52.
