See also
Sepsis
Intravenous fluid
Irradiation of blood products. RCH Blood transfusion service
Central Venous Access Device Management Policy and Procedure (RCH only)
Paediatric Injectable Guidelines
Background to condition
Fever and neutropenia is a common complication of the treatment of cancer. The risk of serious bacterial infection is related to the degree and duration of neutropenia. Bacteraemia is diagnosed in up to one-third of children with FN.
Key points
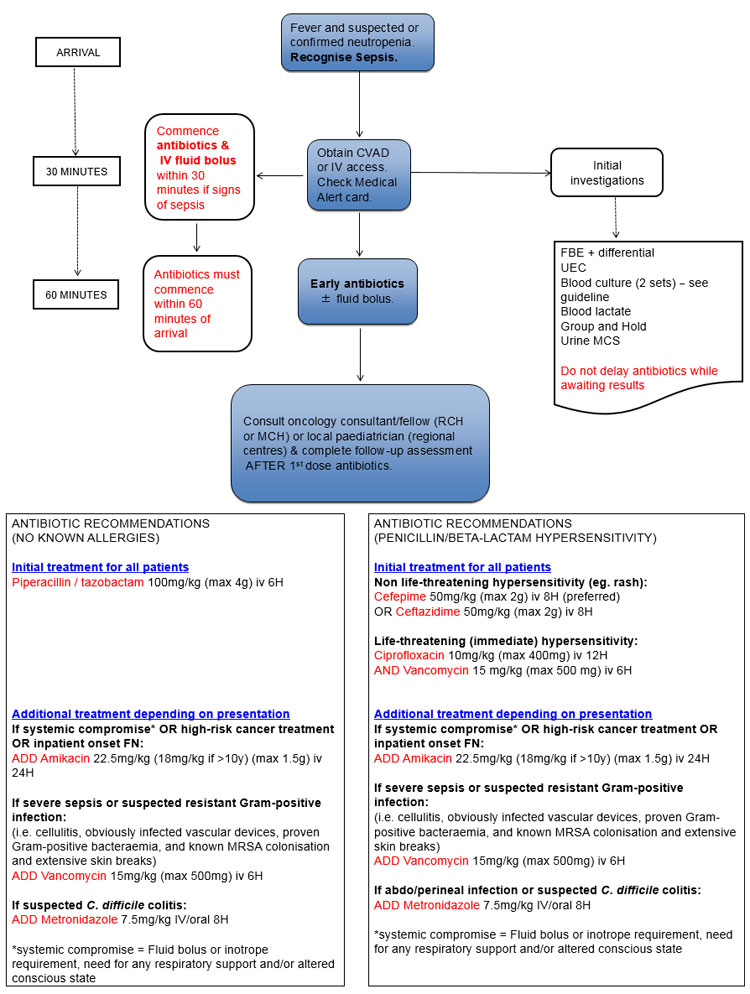
- Fever and suspected or confirmed neutropenia (FN) is a medical emergency.
- Children with FN and signs of sepsis require urgent treatment (Triage Category 2) and those at risk of imminent cardiovascular collapse should be seen immediately (Triage category 1). All other children should commence treatment within 30 minutes of hospital arrival (Triage Category 3).
- Antibiotics must be administered within 30 minutes if there are signs of sepsis and within 60 minutes if there are no signs of sepsis.
- All patients must be discussed with the on-call oncology consultant/fellow (or consultant paediatrician in regional centres) after the 1st dose of intravenous antibiotic.
Definitions
|
Fever |
A single temperature ≥38.5°C or a sustained temperature ≥38.0°C over 1 hour. |
|
Neutropenia |
An absolute neutrophil count
<500/mm3 OR <1000/mm3 with predicted decline to <500/mm3 over the next 48 hours |
|
Suspected neutropenia |
Neutropenia should be suspected in any oncology patient that has received chemotherapy (oral or intravenous) within the last 14 days and other children with recurrent neutropenia. |
|
High risk cancer treatment protocols |
Acute myeloid leukaemia (AML) treatment; Acute lymphoblastic leukaemia (ALL) induction, ALL delayed intensification, infant ALL; lymphoma induction; allogeneic transplant (day-14 to day+356); autologous transplant (day-7 to day +30); re-induction chemotherapy for any relapse. |
|
Systemic compromise |
Fluid bolus or inotrope requirement, need for any respiratory support and/or altered conscious state |
|
Primary Treatment Centre |
The centre where the patient was diagnosed and the treatment plan decided. For the majority of children this will be either Royal Children's Hospital or Monash Children's Hospital. |
Initial assessment and management
Initial treatment algorithm
Request to see patient's Febrile Neutropenia Treatment Card or Letter. This will have important information regarding cancer diagnosis, risk status and recommended antibiotics
History and examination with particular attention to:
- Recognition of sepsis (see
Sepsis)
- Source of infection - children with neutropenia may not have visible signs of infection
Prompt CVAD or IV access (within 30 minutes of arrival):
- Central venous access devices (CVAD) should be accessed by appropriately trained staff for blood sampling and antibiotics. If there is a delay in accessing the CVAD, a peripheral intravenous line should be inserted
- If patient has signs of sepsis, do not delay CVAD or IV access while waiting for anaesthetic cream (ie, EMLA or AnGel) to work
- CVAD access difficulties:
- Regional centres – contact local consultant paediatrician or RCH Children's Cancer Centre AUM (phone advice only)
- RCH and Monash Children's Hospital –
contact details below
- See also
CVAD management policy
Investigations:
- FBE & differential (do not delay antibiotics while awaiting neutrophil count)
- Electrolytes and creatinine
-
Blood cultures (2 sets) - see CCC BC algorithm for details
- Group and hold
- Venous blood gas and lactate
- Urine for M/C/S (do not delay antibiotics while awaiting specimen)
Initial antibiotics (within 30-60 minutes of arrival):
- All patients must be given the first antibiotic within 60 minutes (30 minutes if systemic compromise/sepsis)
- The first antibiotic should be the most broad-spectrum (ie Piperacillin-Tazobactam)
- Patients with a double lumen CVAD should have antibiotics divided and administered down both lumens (preferred option). If not possible, doses should be alternated between the lumens.
- Detailed antibiotic recommendations (including doses) – see below table
|
Patient group |
Recommended antibiotics |
|
Patients who are clinically stable |
Piperacillin-tazobactam* 100 mg/kg (max 4 g) iv 6H |
|
Non-life threatening penicillin hypersensitivity (rash):
Cefepime 50 mg/kg (max 2 g) iv 8H (preferred)
OR Ceftazidime** 50 mg/kg (max 2 g) iv 8H |
|
Life-threatening (immediate) penicillin or beta-lactam hypersensitivity:
Ciprofloxacin 10 mg/kg (max 400 mg) iv 12H
AND Vancomycin 15 mg/kg (max 500 mg) iv 6H (target trough 10-15 mg/L pre 5th dose) |
|
Patients who have any of:
(i) Systemic compromise/sepsis
(ii)
High risk cancer treatment
(ii) Inpatient onset FN |
As for patients who are clinically stable
AND Amikacin*** 22.5 mg/kg (18 mg/kg if >10y) (max 1.5 g) iv 24H
(Target trough
<2 mg/L pre 3rd dose)
Regional centres may choose alternate aminoglycoside (ie gentamicin) depending on local susceptibility data |
|
Patients with suspected resistant Gram-positive infection
(Including patients with cellulitis, obviously infected vascular devices, proven Gram-positive bacteraemia, and known MRSA colonisation with extensive skin breaks) |
As for patients who are clinically stable
AND vancomycin 15 mg/kg (max 500 mg) iv 6H (target trough 10-15 mg/L pre 5th dose)
Cease if susceptibilities indicate an alternative agent can be used
NB. Prolonged fever in a clinically stable patient is NOT an indication to commence vancomycin |
|
Patients with features of abdominal or perineal infection |
As for patients who are clinically stable
AND metronidazole 7.5 mg/kg (max 500 mg) iv/oral 8H if receiving cefepime, ceftazidime or ciprofloxacin as first line antibiotic
NB. Piperacillin-tazobactam will provide adequate anaerobic cover, other than for suspected Clostridium difficile colitis |
* Trade names for piperacillin-tazobactam include Tazocin®, Tazopip®, PiperTaz®, and DBL Piperacillin and Tazobactam®. Doses refer to piperacillin component.
** Ceftazidime has reduced activity against viridans group streptococci. It should be avoided in patients with extensive mucositis, recent high dose methotrexate or cytarabine and ciprofloxacin prophylaxis.
*** IV aminoglycoside antibiotics (ie amikacin and gentamicin) should not be given simultaneously through the same line as IV penicillins including piperacillin-tazobactam and ticarcillin-clavulanate. The line should be flushed well with sodium chloride 0.9%, before and after giving each medication.
Fluid resuscitation
If patient has signs of sepsis:
- Give initial 20 mL/kg Normal Saline IV bolus over a maximum of 10 minutes (not through an infusion pump)
- Monitor for improvement in vital signs, perfusion and/or conscious state
- If only transient improvement occurs, consider additional fluid boluses to a maximum volume of 40 mL/kg
- Total volumes >40 mL/kg should be discussed with senior clinician
- If no improvement, consider inotropic support (see
Sepsis)
Follow-up assessment
After the first dose of antibiotic has been given, complete the following:
A full examination with particular attention to:
- Upper respiratory tract for otitis media and sinusitis
- Oropharynx for dental abscess and mucositis
- Lower respiratory tract for signs of pneumonia, including Pneumocystis jirovecii (PJP) pneumonia (cough, tachypnoea, hypoxia, interstitial infiltrate on CXR)
- Abdomen for signs of Clostridium difficile colitis (generalised abdominal tenderness) or typhlitis (tenderness over caecum)
- Skin for cellulitis or vesicular lesions
- Perineum and perianal area for anal fissure, cellulitis or abscess
- CVAD for signs of tunnel/exit site infection
- Signs of anaemia and/or thrombocytopenia
Additional investigations as indicated:
- Respiratory symptoms:
- CXR (there may be no changes while neutropenic)
- Nasal swab (throat swab if thrombocytopenic), for respiratory virus PCR (only if requested by oncology team after review). Patient placement should NOT be delayed whilst waiting for results (see NPA)
- Sputum for M/C/S in older children
- Diarrhoea:
- Stool for M/C/S and viral studies
- Stool for C. difficile toxin assay if recent treatment with antibiotics
- Skin, CVAD site or mouth lesions:
- Bacterial swab for M/C/S (including Gram stain slide)
- Viral swab of vesicular lesions and mouth ulcers for HSV and VZV PCR
- CNS symptoms
- CT brain and lumbar puncture may be indicated if there are new CNS symptoms or signs. Please discuss with on call oncologist first.
- Correction of thrombocytopenia and/or coagulopathy must occur prior to LP.
- Other
Referral
- All patients presenting to the Emergency Department with FN must be discussed with the local paediatric team (regional centres only) and the treating oncology team (all centres). To avoid antibiotic delays, this should be done after the 1st dose of antibiotic has been given.
- Regional centres - contact the local consultant paediatrician on call after the first dose of antibiotic has been given. Daily consultation with the treating oncology team to discuss ongoing care is also recommended.
- RCH and Monash Children's hospital - contact the treating oncology team after the first dose of antibiotic has been given (see
below for contact details)
- Unless the patient is suitable for discharge, arrange urgent admission to the ward.
Consider transfer to the patient's Primary Treatment Centre (PTC) if:
- Severe sepsis
- Clinical deterioration despite broad spectrum antibiotics
- Prolonged (>72 hours) FN despite broad spectrum antibiotics
Transfer should be discussed with the oncology consultant/fellow at the patient's primary treatment centre (see
contact details below).
For emergency advice and paediatric or neonatal ICU transfers, call the Paediatric Infant Perinatal Emergency Retrieval (PIPER) Service: 1300 137 650.
Ongoing management (beyond the 1st 24 hours)
Clinical assessment
Patients should undergo daily:
- Examination for signs of an infective focus
- Review of microbiology results to guide antibiotic therapy
- Routine daily blood cultures are not recommended in patients who are well and afebrile. A blood culture set should only be repeated on Day 2 (+/- Day 3) in patients who remain febrile. A repeat blood culture set is also indicated for any of: (i) new onset fever or clinical instability (after afebrile >48h); (ii) change to antibiotics or; (iii) to confirm/exclude a positive blood culture result.
Modifying antibiotics
After 24-48 hours, discontinue Amikacin and/or Vancomycin (if initiated) unless
- patient is clinically unstable (additional or alternate antimicrobials may be required: discuss with Infectious Diseases) or
- a resistant organism has been identified that requires Amikacin or Vancomyin (Discuss with Infectious Diseases)
Do not broaden initial empiric antibiotic regimen based solely on persistent fever in children who are clinically stable
All treatment modifications, including cessation of antibiotics, should be discussed with the patient's primary oncology team.
Stopping antibiotics
All patients:
- Evidence of marrow recovery – Discontinue empiric antibiotics in patients who have negative blood cultures at 48 hours and who have been afebrile for at least 24 hours
- No evidence of marrow recovery and neutropenia expected to be prolonged – The decision to discontinue or continue antibiotic therapy should be made by the primary oncologist taking into account the intensity of recent chemotherapy and associated risk factors. Cessation of antibiotic therapy may be considered if (i) cultures are negative; (ii) skin and mucous membranes are intact and (iii) there are no impending invasive procedures or ablative chemotherapy planned.
Low-risk FN:
- Consider discontinuation of empiric antibiotics at 72 hours in low-risk patients (as judged by the treating oncologist) who have negative blood cultures and who have been afebrile for at least 24 hours, irrespective of marrow recovery status, provided careful follow-up is ensured
All treatment modifications, including cessation of antibiotics, should be discussed with the patient's primary oncology team
Prolonged (>72 hours) or recurrent fever despite broad-spectrum antibiotic/s
- Evaluate and consider treatment for invasive fungal infection (IFI) (see below)
- Regional centres should transfer patients with prolonged or recurrent fever to their primary treatment centre for further investigations
- Patients considered high-risk for IFI include those with (i) relapsed acute leukemia; (ii) AML; (iii) Graft versus Host Disease (GvHD); (iv) allogeneic HSCT; (v) severe aplastic anaemia; (vi) prolonged corticosteroid use and (vii) prolonged ICU admission. All other patients should be categorised as low-risk for IFI (please note that low-risk does not equal no-risk)
Evaluation for IFI
- CT of lungs and CT sinuses (age >2y) and targeted imaging of other clinically suspected areas of infection
- Bronchoscopy and lavage (BAL) if pulmonary infiltrates detected on CT lungs (consult local Infectious Diseases Unit to ensure appropriate investigations on BAL fluid are done)
- Fungal cultures from blood, BAL and other sterile sites as indicated
- Galactomannan (+/- aspergillus PCR if available) on blood and BAL fluid
Empiric treatment for suspected IFI
- Consult local Infectious Diseases Unit
- Recommended empiric antifungal agent is amphotericin (caspofungin or voriconazole if amphotericin contraindicated). For targeted IFI treatment, discuss with local Infectious Diseases Unit.
- IFI high risk – initiate empiric antifungal treatment for prolonged or recurrent fever of unclear etiology that is unresponsive to broad-spectrum antibacterial agents
- IFI low risk – consider empiric antifungal therapy in setting of persistent FN
Information specific to RCH
Information specific to Monash Children's Hospital Low risk FN program - policy For
referral or discussion of patients with FN
- Business hours
- Weekdays 0900 – 1700:
Contact the Paediatric Oncology Fellow on Ascom (03)
8572 4245 or via MCH switch (03) 9594 6666 -
After hours - including weekends, overnight
and public holidays:
Contact the Paediatric Oncologist on call via MCH switch (03)
9594 6666
For CVAD access issues - Business hours
- Weekdays 0800 – 1800:
Contact the 4C Reef, Children's Cancer Centre ANUM on Ascom
(03) 8572 4242 or 0417 243 401 - After
hours - including weekends, overnight and public holidays
Contact the
4A Canopy ANUM on (03) 8572 4071 or
(03) 8572 3400
|